While antidepressant medications remain the most common first-line treatment for depression, research shows they only work for about one-third of patients and often come with unpleasant side effects like headaches, digestive problems, and brain fog.
For those with major depressive disorder (MDD) or treatment-resistant depression, brain stimulation therapies offer an alternative path to relief.
This article covers:
-
The various brain stimulation therapies available today, including how they work, their effectiveness, potential side effects, and costs.
-
A comparison of these treatments to help you determine the best option for you or a loved one.
-
Why fMRI-guided TMS (following the “SAINT protocol”) is the best overall treatment option.
Table of Contents
What Are Brain Stimulation Therapies?
Brain stimulation therapies play an important role in treating mental disorders like depression. These therapies modify brain activity through electrical or magnetic stimulation.
Some, such as electroconvulsive therapy (ECT) and vagus nerve stimulation (VNS), use direct electrical currents applied internally or externally, while others, like transcranial magnetic stimulation (TMS), rely on magnetic fields to stimulate neural activity.
Doctors typically recommend these treatments for patients with severe depression or those who have not responded to psychotherapy or medication.
Next, we’ll dive into these therapies, starting with one of the most widely used options: TMS.
Transcranial Magnetic Stimulation (TMS)
What Is TMS?
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation therapy that uses an electromagnetic coil to deliver short, low-intensity magnetic pulses to the brain. These pulses create weak electrical currents that stimulate neurons and neural circuits in regions affected by depression.
This therapy specifically targets the dorsolateral prefrontal cortex (DLPFC), an area of the brain crucial for mood regulation, motivation, and reward processing. In individuals with depression, activity in this region is often reduced, and TMS helps restore normal function.
How Does TMS Work?
The U.S. Food and Drug Administration (FDA) approved TMS in 2008 for patients with treatment-resistant depression — those who do not respond to antidepressant medication. The most common form, repetitive transcranial magnetic stimulation (rTMS), remains widely used today. Since its approval, TMS has also been cleared to treat depression with comorbid anxiety and depression with suicidality.
A standard rTMS session lasts about 40 minutes, with patients undergoing daily treatments five days a week for 4–6 weeks (totaling 20–30 sessions). Some experience improvement within 2–4 weeks, while others notice benefits only after completing treatment.
To reduce the time required to complete a course of treatment, researchers developed Intermittent Theta-Burst Stimulation (iTBS), which the FDA approved in 2018. iTBS mimics theta brain waves to enhance neural connectivity, using a rapid triple-pulse pattern.
This innovation reduces session time to just three minutes (compared to 37 minutes for rTMS), allowing for quicker relief. Many patients respond soon after starting, and studies show it may rapidly reduce suicidal ideation.
In 2022, Stanford researchers introduced Stanford Accelerated Intelligent Neuromodulation Therapy (the SAINT protocol), a major advancement in TMS. This method integrates theta-burst stimulation with functional MRI (fMRI) and neuronavigation to precisely target the dorsolateral prefrontal cortex (DLPFC).
By personalizing coil placement for each patient, SAINT achieves higher accuracy and effectiveness, making it the gold standard for treatment-resistant depression.
Effectiveness of TMS
Repetitive TMS (rTMS) improves depression symptoms in ~50% of patients, with 30% achieving remission. When combined with psychotherapy, success rates increase to 66% response and 55% remission.
Accelerated TMS and iTBS are similarly effective, with ~50% of patients responding and nearly 30% reaching remission. Patients receiving multiple sessions per day often improve faster than those on a standard daily schedule, with benefits lasting for months.
The SAINT protocol delivers even greater outcomes. In a clinical trial, 85% of patients responded, and 78% achieved remission, despite having treatment-resistant depression. One month post-treatment, 60% remained in remission, making SAINT one of the most effective treatments available.
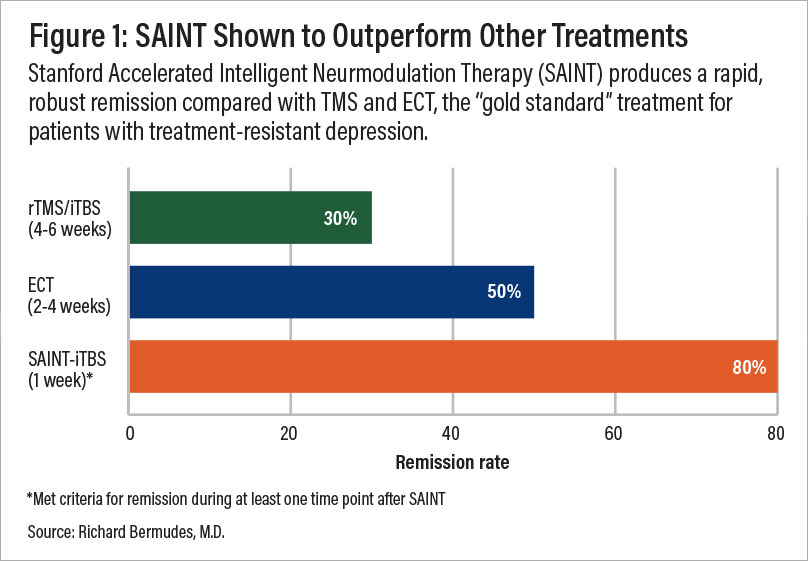
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
Note: Our Utah-based clinic, Cognitive FX, is one of fewer than 10 clinics in the U.S. currently offering fMRI-guided TMS treatment following the SAINT protocol. Click here to learn more and see if you’re a good fit for treatment.
Side Effects of TMS
TMS is safe and well tolerated, with most patients only experiencing mild, short-lived side effects. These typically resolve within minutes after a session and can be managed with over-the-counter pain relievers.
Common side effects include:
- Discomfort at the magnet site
- Tingling or muscle contractions in the scalp, jaw, or face
- Mild headaches
- Lightheadedness or dizziness
- Sensitivity to sound
The most serious, though extremely rare, side effect is a seizure, occurring in less than 0.01% of sessions (less than 1 in 10,000).
Cost of TMS Treatment
TMS costs $6,000–$15,000 per treatment course, depending on location, provider expertise, TMS type, and additional services (e.g. medical evaluations or psychotherapy). Clinics with advanced technology may charge higher fees.
Insurance may cover TMS, but patients often must first try 2–4 antidepressant medications and/or therapy to qualify. Coverage varies by provider and policy.
Currently, while FDA-approved, fMRI-guided TMS (the SAINT Protocol) is not covered by insurance.
Electroconvulsive Therapy (ECT)
What Is ECT?
Electroconvulsive therapy (ECT) is a noninvasive treatment that uses electric currents to induce controlled seizures in the brain, effectively treating severe mental health disorders. It has the longest history of use for depression and remains one of the most widely used brain stimulation therapies.
The FDA approves ECT for major depression, severe depressive episodes, catatonia, and treatment-resistant depression, particularly in suicidal or high-risk patients. Unlike antidepressants, ECT works quickly, often producing noticeable improvement within a week.
How Does ECT Work?
Before treatment, patients receive general anesthesia and a muscle relaxant to prevent movement. Electrodes are then placed on the head, delivering an electric current that induces a brief seizure (under a minute). After waking from anesthesia, patients may feel groggy but typically recover within an hour and resume normal activities.
ECT is typically administered three times a week for 6–12 weeks. To prevent relapse, most patients require ongoing treatment, such as maintenance ECT, antidepressants, or both, with sessions ranging from weekly to every few months, depending on individual needs.
Effectiveness of ECT
ECT is often more effective than antidepressants for treating depression. Studies show that over 50% of patients improve within the first week, with nearly 80% experiencing significant symptom relief. Remission rates range from 40–60% after several weeks of treatment.
ECT is particularly effective for patients with suicidal ideation or those who haven’t undergone multiple rounds of antidepressant therapy. While the exact number of sessions needed to stop intrusive thoughts varies, most patients see early improvement in their symptoms.
To prevent relapse, patients typically continue maintenance ECT, antidepressants, psychotherapy, or a combination of these. Abruptly stopping ECT leads to relapse in over 80% of cases, so ongoing sessions — starting weekly and gradually decreasing over time — are recommended. Combining ECT with antidepressants reduces relapse risk by 50% in the first year.
Side Effects of ECT
The most common side effects associated with ECT include:
- Headaches
- Upset stomach and nausea
- Muscle aches and pains
- Dizziness
- Memory loss
- Disorientation or confusion
- Cognitive issues
Memory issues are more common with bilateral ECT, where electrodes are placed on both sides of the head. Unilateral ECT, which uses a single electrode on the right side, carries a lower risk of memory loss because it avoids stimulating areas involved in learning and memory.
Cost of ECT Treatment
ECT costs $300–$1,000 per session, with initial treatment requiring 5–15 sessions and 10–20 maintenance sessions annually. This can bring the total yearly cost to over $10,000.
Despite the expense, studies show ECT is cost-effective due to its higher success rates compared to antidepressants. Many insurance plans cover psychiatric treatments, often providing partial reimbursement for ECT.
Vagus Nerve Stimulation (VNS)
What Is VNS?
Vagus nerve stimulation was initially developed as a treatment for epilepsy, but in 2005, the FDA approved it to treat depression in patients with severe or recurrent symptoms or treatment-resistant depression.
How Does VNS Work?
Unlike ECT and TMS, vagus nerve stimulation (VNS) requires a minor surgery to implant a watch-sized device in the upper left chest. This device sends electrical pulses through the left vagus nerve, which runs from the brainstem through the neck, chest, and abdomen, delivering signals to the brain. Pulses typically last 30 seconds every 5 minutes, but settings vary per patient. VNS is painless during operation.
A newer, noninvasive version called transcutaneous VNS (tVNS) uses a portable device to stimulate the vagus nerve through the skin. This method is more accessible and affordable, avoiding surgery, but it is not yet FDA-approved for depression. Research is ongoing to evaluate its effectiveness and safety.
Effectiveness of VNS
Although FDA-approved, VNS is not a first-line treatment and is used infrequently due to mixed efficacy results. Response rates range from 15% to 50%, but over 50% of patients relapse within a year. Some see improvements after months, while others experience no benefit or worsening symptoms.
Side Effects of VNS
VNS carries surgical risks, including infection, pain, or device complications that may require additional surgery.
Other potential side effects include:
- Discomfort or tingling at the implant site
- Voice changes or hoarseness
- Cough or sore throat
- Neck pain or headaches
- Breathing problems, especially during exercise
- Difficulty swallowing
- Nausea or vomiting
Cost of VNS Treatment
Costs for a full VNS treatment range between $8,000 and $14,000. Historically, VNS for treatment-resistant depression wasn’t covered by insurance providers, making this option out of reach for most patients. However, in 2019, the Center for Medicare and Medicaid Services started allowing coverage for participants.
Experimental Brain Stimulation Therapies
Other brain stimulation therapies are currently being developed to treat patients with depression, including:
Deep Brain Stimulation (DBS)
Deep brain stimulation (DBS) is an experimental treatment that involves surgically implanting electrodes in specific brain regions to deliver continuous, customized electrical pulses.
Originally developed for movement disorders like Parkinson’s disease, DBS is FDA-approved for OCD and received Breakthrough Device Designation in 2022 for major depressive disorder and treatment-resistant depression. Preliminary results are promising, but more studies are needed before widespread approval for depression.
Transcranial Direct Current Stimulation (tDCS)
Transcranial direct current stimulation (tDCS) is an experimental brain stimulation technique that delivers a weak electrical current through scalp electrodes using a small portable device. Typically, the anode is placed over the left DLPFC and the cathode over the right DLPFC.
In a study of patients with severe depression, ~30% responded to tDCS, with ~20% achieving remission. Despite its lack of FDA approval, tDCS is approved in the EU, Israel, and Singapore.
Other Emerging Therapies
Many other brain stimulation treatments are currently under development, including trigeminal nerve stimulation, temporal interference, andtranscranial random noise stimulation. These treatments are available only as part of clinical trials or research studies, but could become available in the next few years.
Comparing Brain Stimulation Therapies
Choosing the right brain stimulation therapy depends on factors such as effectiveness, speed of results, side effects, cost, and accessibility. The table below provides a concise comparison of the most common options.
Therapy
Type |
Effectiveness |
Speed of Results |
Side
Effects |
Cost & Accessibility |
| TMS (rTMS/iTBS) |
~50% response,
~30% remission |
2-6 weeks (rTMS)
Faster with iTBS |
Mild headaches,
scalp discomfort |
$6,000 - $15,000
Widely available |
SAINT-TMS
(Stanford Accelerated Intelligent
Neuromodulation Therapy) |
85% response,
78% remission |
Rapid
(1st week
of treatment) |
Mild headaches,
temporary scalp
discomfort |
$12,000 - $36,000
~10 clinics in the U.S. |
Electroconvulsive Therapy
(ECT) |
~80% response,
~40-60% remission |
Rapid
(1st week
of treatment) |
Short-term memory loss,
headaches |
$300 - $1,000 per session
Available in hospitals |
Vagus Nerve Stimulation
(VNS) |
15-50% response,
high relapse rate |
Takes months
to see results |
Surgical risks, voice changes, nausea |
$8,000 - $14,000
Less common |
Deep Brain Stimulation
(DBS)
*Experimental |
Promising
(still under study) |
Varies per patient |
Surgical risks,
potential brain damage |
High $50,000+
(if approved)
Limited to clinical trials |
Transcranial
Direct Current
Stimulation
(tDCS)
*Experimental |
~30% response,
~20% remission |
Gradual improvement over weeks |
Mild scalp irritation, dizziness |
TBD
Not FDA-approved |
Key Takeaways
-
Fastest results: SAINT-TMS and ECT provide the quickest relief, making them ideal for severe cases.
-
Best overall effectiveness: SAINT-TMS has the highest success rates while remaining noninvasive and well-tolerated.
-
Most widely available: rTMS and ECT are the most accessible treatments, with some insurance coverage.
-
Experimental: DBS and tDCS are still in development, with uncertain long-term effectiveness.
Why fMRI-Guided TMS (SAINT Protocol) Stands Out
At Cognitive FX, we provide fMRI-guided TMS following the SAINT protocol. We believe this method is superior to other brain stimulation techniques currently available.
SAINT is:
-
Accurate and reliable: SAINT uses functional MRI to pinpoint the exact target location for each patient, combined with a neuronavigation system to allow operators to place the target over the same location consistently every session. No other brain stimulation treatments offer anything similar.
-
Fast: SAINT uses iTBS, condensing the treatment into a single week. This makes it much more convenient for patients to fit into their lives and offers faster relief. Patients sit for 10 short sessions each day, for 5 days, with a 50-minute break between sessions. In contrast, other brain stimulation treatments take weeks to complete and are more disruptive for the patient.
-
Effective: With 85% response rate and 78% remission rate, SAINT boasts the highest efficacy of all current brain stimulation techniques.
-
Safe: SAINT is safe and well-tolerated. Patients only experience mild and short-lived side effects which dissipate after a few sessions. This contrasts with some other brain stimulation treatments, especially ECT, where patients may experience memory loss and other cognitive issues.
Receiving Treatment at Cognitive FX
To improve long-term outcomes for our patients, we incorporate cognitive behavioral therapy (CBT) into our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively, in the short term. We expect that adding CBT to the SAINT protocol will improve both initial and longer-term outcomes, as it significantly increases the durability of effects after treatment ends.
Our one-week program is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or those who are actively suicidal and in need of crisis care.
If you are interested in receiving TMS therapy at Cognitive FX, click here to learn more.
Cited Research