How Long Has TMS Been Around?
While many people are learning about Transcranial Magnetic Stimulation (TMS) for the first time, this brain stimulation method has been helping patients for nearly 40 years. Originally developed as...

Transcranial magnetic stimulation (TMS) has been FDA-approved for treating major depressive disorder (MDD) since 2008 and is a well-established treatment option, especially for patients who haven’t responded to antidepressant medications.
However, the standard protocol — repetitive transcranial magnetic stimulation (rTMS) — has notable limitations:
Lengthy treatment: rTMS requires daily sessions over 4–6 weeks, making it difficult to complete alongside work and life commitments.
Lack of precision: Coil placement relies on manual measurement, leading to inconsistencies affecting treatment effectiveness.
Moderate success rates: While rTMS has higher response and remission rates than antidepressant medications, only about one-third of patients achieve full remission.
To address these challenges, Nolan Williams and his team at Stanford University developed a faster, more effective TMS approach called Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT™ or SNT).
In this article, we’ll discuss how SAINT™ differs from conventional TMS and why it may be a breakthrough option for you.
We cover:
Approved in 2022 by the U.S. Food and Drug Administration for treating treatment-resistant depression, SAINT™ is currently the safest and most efficient type of TMS. Treatment resistance is defined as a lack of improvement after multiple interventions, including antidepressant medications and even electroconvulsive therapy (ECT).
Like all forms of TMS, SAINT uses a magnetic coil placed over the patient’s head to stimulate neuronal activity. The coil generates low-frequency magnetic pulses to regulate activity in the dorsolateral prefrontal cortex (DLPFC), a region of the brain crucial for mood regulation.
SAINT differs from conventional TMS in three key ways:
Use of intermittent theta-burst stimulation (iTBS)
SAINT TMS utilizes intermittent theta-burst stimulation, a form of brain stimulation that uses a different pulse frequency and pattern, delivering treatment in shorter sessions. The SAINT treatment protocol consists of 10-minute sessions, compared to the 37-minute sessions of rTMS.
Precise targeting with fMRI and neuronavigation
One of the key distinctions of the SAINT protocol is the use of neuroimaging and neuronavigation for precision targeting. A functional MRI scan locates the target area of the brain (the dorsolateral prefrontal cortex, or DLPFC), while neuronavigation ensures the magnetic coil is placed accurately for every treatment session.
This precise targeting is vital for effectiveness, as even a slight misplacement of the coil can diminish results. In contrast, other TMS methods, like rTMS and standard iTBS, often rely on less precise methods for coil placement, such as manual measurements taken over the patient’s scalp.
Condensed treatment schedule
SAINT TMS is administered over a condensed one-week period, with 10 short sessions per day for 5 consecutive days, totaling 50 sessions.
This compressed treatment schedule makes it more convenient for patients who might find it challenging to commit to the longer durations of other TMS protocols, such as the 4–6 week timeframe of rTMS.
These innovations make SAINT faster, more precise, and more effective than conventional TMS.
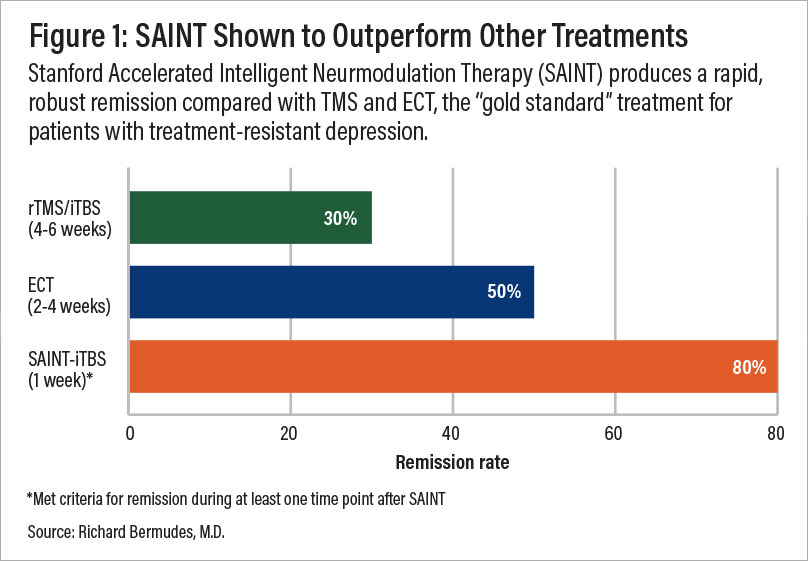
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
SAINT TMS is considered the "gold standard" treatment for treatment-resistant depression. Clinical trials showing SAINT TMS to be highly effective include:
High response rate: In a double-blind, randomized controlled trial, about 85.7% of patients responded to SAINT treatment, meeting pre-defined criteria for a reduction in depressive symptoms.
High remission rate: 78.6% of patients in the same trial met the criteria for remission, indicating a significant decrease or disappearance of depression signs and symptoms.
Rapid-acting: These results were achieved in just one week of treatment, making SAINT one of the fastest-acting depression treatment options available. A month after treatment, 60% of trial participants remained in remission.
While the original form of TMS can improve symptoms in about 50% of patients, with over 30% achieving remission, SAINT boasts significantly higher success rates. With SAINT, 90% of patients who previously didn’t respond to conventional TMS achieved remission in just three to five days.
Note: Learn more about how SAINT TMS compares to ECT and ketamine therapy.
SAINT is a safe, noninvasive, outpatient treatment, with most patients experiencing only mild and short-lived side effects.
As with all forms of TMS, the most common side effects include headaches, dizziness, and mild discomfort at the stimulation site or in the facial muscles during treatment.
Less common side effects — though still generally mild — may include gastrointestinal discomfort, muscle twitching, insomnia, tinnitus, or fainting. These effects typically subside within the first few sessions and can often be managed with over-the-counter pain relievers.
One key advantage of SAINT over traditional TMS is its lower risk of seizures. While the seizure risk with standard TMS is already low (fewer than 3 cases per 100,000 sessions), SAINT further reduces this risk by using a lower stimulation intensity — 80% of the brain’s motor threshold compared to 110%–120% used in rTMS. This adjustment makes SAINT an even safer option for patients.
If you’ve been dealing with treatment-resistant depression and other treatments have failed to work or produced unpleasant side effects, SAINT treatment could be a great option for you.
However, while TMS is safe and effective for most patients with major depression, certain medical conditions may make it unsuitable, including:
Metal implants in the head: TMS is not recommended for patients with metal implants near the treatment area, such as cochlear implants, internal pulse generators, medication pumps, aneurysm clips or coils, stents, or bullet fragments. While braces and dental fillings are safe, metal objects in the brain can overheat or malfunction due to the magnetic field, posing serious risks.
History of seizures: Though rare, seizures can occur during TMS, especially in individuals with epilepsy, multiple sclerosis, Alzheimer’s disease, or a history of seizures. Discussing your medical history and medications with a doctor can help minimize risks.
Certain prescription medications: Some medications may increase seizure risk. While no direct evidence suggests TMS significantly raises this risk, patients on such medications should consult their healthcare provider to determine if adjustments or closer monitoring are needed.
If you have any of these conditions or concerns, it's crucial to consult with a healthcare professional. They can assess your medical history, current condition, and individual needs to determine if SAINT TMS is right for you.
Beyond medical factors, some patients may also face practical barriers to treatment, such as insurance coverage limitations, cost, or geographical constraints. If these apply to you, discussing alternative options with your provider may help you explore solutions.
While SAINT offers significant advantages over traditional TMS, the following may make it less accessible:
Lack of insurance coverage: Most insurance plans do not yet cover SAINT, as it is a newer protocol compared to standard TMS. However, coverage policies vary, so it’s important to check with your insurance provider to confirm your options. The Centers for Medicare and Medicaid Services (CMS) have announced that hospitals will be reimbursed $19,703 for the full SAINT™ protocol, beginning July 1, 2025.
High treatment costs: Currently, SAINT TMS treatments range from $30,000 to $36,000, making the cost a significant barrier for many patients. There are, however, close alternatives available at a significantly lower price (discussed below).
Limited availability: Fewer than 10 clinics in the U.S. currently offer SAINT, with most providers still using rTMS. However, more clinics are beginning to adopt accelerated TMS and intermittent theta burst stimulation (iTBS) protocols, signaling a gradual shift toward newer treatment methods.
If you’re considering SAINT, discussing financial and logistical concerns with your provider can help you explore potential solutions, such as financing options or alternative treatment locations.
Our clinic, based in Provo, Utah, provides an alternative to SAINT TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT™ (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
| Accelerated fMRI - TMS | Magnus SAINT™ TMS | |
|---|---|---|
| FDA-Approved iTBS | ✔ | ✔ |
| FDA-Approved Neuronavigators | ✔ | ✔ |
| FDA-Approved Figure 8 Coils | ✔ | ✔ |
| Number of Treatment Days | 5 | 5 |
| Treatments per Day | 10 | 10 |
| Total Treatments | 50 | 50 |
| Number of TMS Pulses | Approx. 90,000 | 90,000 |
| Resting motor threshold pulse intensity | 90–120% | 90–120% |
| FDA-Approved Personalized DLPFC Targeting | ✘ | ✔ |
| Personalized DLPFC Targeting Assists Doctor in Target Location | ✔ | ✘ |
| Personalized E Field Coil orientation | ✔ | ✘ |
| Cost | $9,000 to $12,000 | $30,000+ |
DISCLAIMER: SAINT™ is a trademark of The Board of Trustees of the Leland Stanford Junior University (“Stanford”) and has exclusively licensed such mark to Magnus Medical. Cognitive FX is neither endorsed by Stanford nor utilizes Magnus Medical equipment nor claims to be offering the SAINT™ protocol as prescribed by Stanford University et. al. or Magnus Medical. We provide fMRI-guided intermittent theta burst TMS with target locations determined by fMRI and our prescribing physician.
As we’ve highlighted throughout this article, this protocol of TMS is:
Safe: Widely tolerated and associated with mild, short-lasting side effects.
Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work commitments (compared to 4 to 6 weeks of standard TMS and acceleratedTMS protocols).
Effective: Precision coil placement combined with theta burst stimulation produces the best TMS treatment results to date.
To improve outcomes for our patients, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.

Dr. Mark D. Allen holds a Ph.D. in Cognitive Science from Johns Hopkins University and received post-doctoral training in Cognitive Neuroscience and Functional Neuroimaging at the University of Washington. As a co-founder of Cognitive Fx, he played a pivotal role in establishing the unique and exceptional treatment approach. Dr. Allen is renowned for his pioneering work in adapting fMRI for clinical use. His contributions encompass neuroimaging biomarkers development for post-concussion diagnosis and innovative research into the pathophysiology of chronic post-concussion symptoms. He's conducted over 10,000 individualized fMRI patient assessments and crafted a high-intensity interval training program for neuronal and cerebrovascular recovery. Dr. Allen has also co-engineered a machine learning-based neuroanatomical discovery tool and advanced fMRI analysis techniques, ensuring more reliable analysis for concussion patients.

While many people are learning about Transcranial Magnetic Stimulation (TMS) for the first time, this brain stimulation method has been helping patients for nearly 40 years. Originally developed as...

If you’re considering brain stimulation therapy for treating major depression, you may wish to understand the differences between repetitive transcranial magnetic stimulation (rTMS) and the more...

If you’re considering brain stimulation therapy for treating major depression, you may wish to understand the differences between repetitive transcranial magnetic stimulation (rTMS) and deep...
In general, increased anxiety is not a common side effect of transcranial magnetic stimulation (TMS), though some patients have experienced a temporary increase in anxiety during or after treatment.
Although transcranial magnetic stimulation (TMS) has been used for over 20 years and FDA-approved for treating major depressive disorder since 2008, it remains relatively less well-known among the...

For many people living with major depression, antidepressant medications either don’t work or only get them part of the way to recovery. Symptoms may ease for a while, but then return, or never fully...