With traditional antidepressant medications only working effectively for about one-third of patients, researchers and clinicians are continuously developing new treatment options for treatment-resistant depression.
Ketamine therapy and transcranial magnetic stimulation (TMS) are two of the leading alternatives. Both are FDA-approved and worth considering as they’ve been shown to relieve symptoms (at least temporarily) in a significant number of patients.
With that said, these treatments are distinctly different. They each come with a different set of side effects and have varying short and long-term treatment outcomes.
In this article, we provide a detailed comparison of these two treatments to educate potential patients about the differences and help them decide which may be the best for them.
We compare TMS and ketamine in terms of:
- How they work
- Common side effects
- How effective they are
- Cost and insurance coverage
Note: In addition, we also compare TMS and ketamine to esketamine nasal spray (a related but chemically different compound that is also FDA-approved for the treatment of depression).
Transcranial Magnetic Stimulation (TMS)
Transcranial Magnetic Stimulation (TMS) uses an electromagnetic coil to apply a magnetic pulse directly to a part of the brain called the dorsolateral prefrontal cortex (DLPFC). This region is associated with our ability to feel positive feelings, including reward and motivation, and is often impacted by depression.
How Does TMS Work?
During a TMS treatment session, the electromagnetic coil is positioned directly above the DLPFC, and magnetic pulses are delivered at specific intervals to stimulate the nerve cells in that area. The aim is to increase activity in that region which, in turn, can alleviate symptoms in depressed patients.
The original method of TMS (called repetitive transcranial magnetic stimulation or rTMS) is administered once a day for about 30 minutes, five days a week for four to six weeks. However, due to the extensive time commitment required from patients, researchers developed an accelerated TMS protocol with multiple daily sessions, shortening the course of treatment to just one week. This condensed time-frame makes it easier for patients to complete the treatment.
Advancements in TMS Improve Treatment Outcomes and Patient Experiences
In 2018, the FDA approved a novel form of accelerated TMS known as Intermittent Theta-Burst Stimulation (iTBS). iTBS employs a different magnetic pulse pattern (delivered in triplets) and administers treatment in just three minutes, compared to the 37 minutes required for rTMS treatment. Many patients notice improvements soon after beginning treatment. Additionally, there is a reduction in suicidal ideation, indicating that iTBS may be a rapid treatment option for patients at high risk of suicide.
Building on this, researchers at Stanford combined iTBS with functional MRI and neuronavigation to ensure precise coil placement over the specific area of the brain targeted by TMS. The SAINT™ protocol received FDA-clearance in September 2022 and is now considered the “gold standard” for treating treatment-resistant depression.
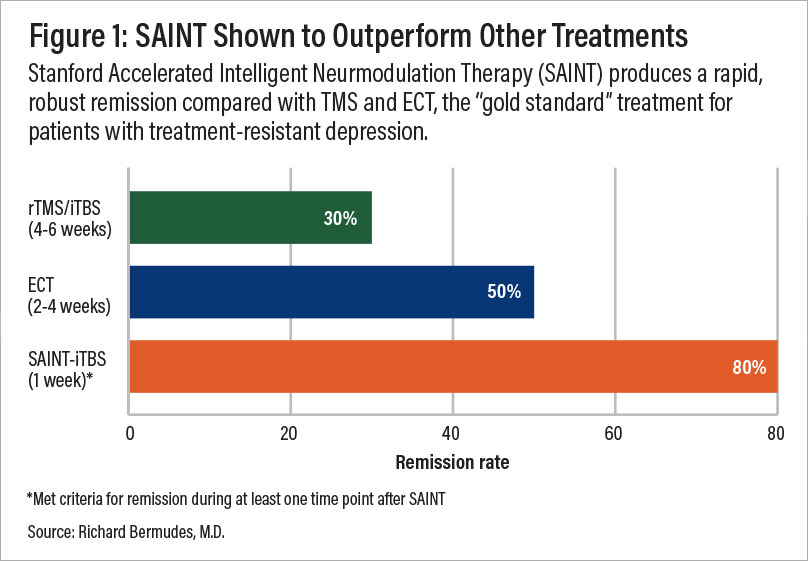
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
Key differences between conventional rTMS and SAINT TMS include:
- Duration: 5-day treatment with SAINT vs. four to six weeks with conventional rTMS.
- Precision: fMRI and neuronavigation used in SAINT ensure precise coil placement compared to manual measurements used in rTMS.
- Speed to results: SAINT is faster-acting compared to conventional rTMS with many patients seeing response and remission in a single week.
Side Effects of TMS
TMS is a safe and well-tolerated procedure with mild and short-lived side effects. The most common side effects include headaches, dizziness, and some discomfort at both the stimulation site and in the facial muscles during treatment.
The most serious potential side effect of TMS is a seizure, but these are extremely rare. The risk of having a seizure from a TMS session is less than 0.01% per session or less than 1 in 10,000 sessions.
How Effective Are TMS Treatments?
The original form of TMS, rTMS, has been shown to improve depression symptoms in about 50% of patients, with over 30% achieving remission. When combined with psychotherapy, success rates are even more impressive with remission and response rates of ~55% and ~66% respectively.
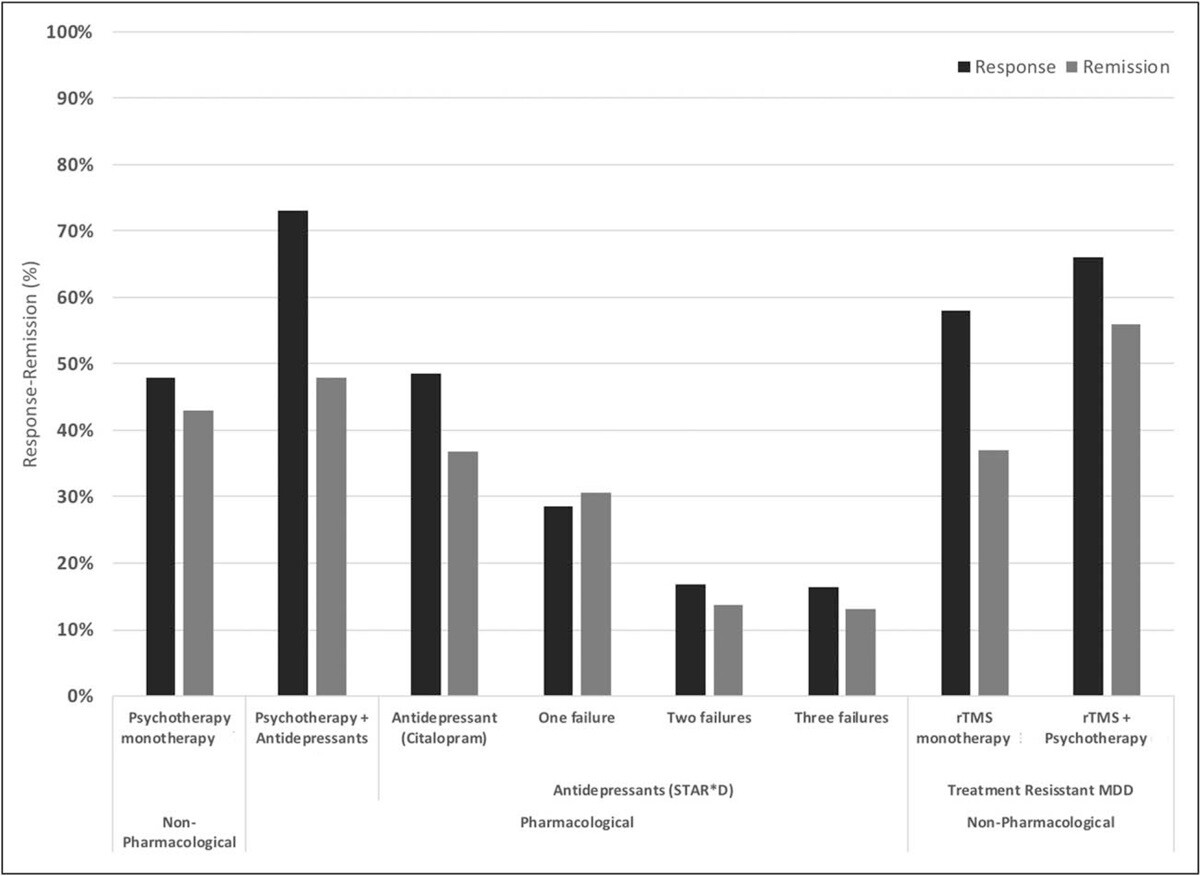
Response and remission rates of various monotherapeutic and combinatory antidepressant treatments based on the largest studies and datasets available. [Source]
So far, the team from Stanford published two studies looking at the efficacy of the SAINT™ protocol. The first study, published in April 2020, included patients who previously didn’t respond to conventional TMS and found that 90% achieved remission in as little as three to five days.
The follow-up study, published in October 2021, excluded patients previously treated with TMS and confirmed the positive results: about 85% of patients responded to the treatment, and around 78% achieved remission within five days of treatment. About four weeks later, remission rates dropped somewhat but remained around 60%.
More research is needed to evaluate its long-term durability, but it is clear that fMRI-guided iTBS offers more rapid antidepressant effects compared to conventional TMS and other alternative depression treatments. This combined with minimal side effects makes it one of the most promising treatments available today.
While some patients may experience lasting remission or symptom reduction, others might require periodic follow-up treatments to maintain effectiveness. This seems to help patients remain symptom-free and avoid relapse. As such, combining TMS with cognitive behavioral therapy (CBT), which has been shown to produce strong long-term outcomes, is likely to offer the best results for patients.
How Much Does a TMS Treatment Cost?
The costs associated with TMS sessions vary greatly depending on geographic location, provider expertise, the type of TMS used, and insurance coverage. A typical TMS treatment course ranges from $6,000 to $15,000 without insurance.
Currently, only conventional rTMS is covered by most insurance providers. However, depending on your provider and specific policy, you may need to have tried two to four antidepressant medications and/or therapy with a psychiatry specialist before you can qualify for TMS coverage.
Clinics with advanced technology or offering additional services — such as medical evaluations or talk therapy — may charge higher fees. For example, fMRI-guided protocols such as SAINT™ treatment cost upwards of $30,000.
Receiving Accelerated fMRI-Guided TMS for Depression at Cognitive FX
Our clinic in Provo, Utah, provides an alternative to SAINT™ TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT™ (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
This accelerated protocol of iTBS is:
- Safe: Widely tolerated and associated with mild, short-lasting side effects.
- Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
- Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work commitments (compared to 4 to 6 weeks of standard TMS and accelerated TMS protocols).
To improve patient outcomes, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.
Ketamine
Ketamine is an anesthetic drug with dissociative properties, consisting of two enantiomers: S-ketamine and R-ketamine. These mirror-image chemicals have slightly different effects but are both used in ketamine treatments.
Ketamine is now used to address treatment-resistant depression, suicidal thoughts, and bipolar disorder. However, while ketamine can quickly alleviate depressive symptoms, it should be combined with other treatments, such as antidepressants and psychotherapy, for sustained results.
How Does Ketamine Work?
It’s not known exactly how ketamine helps patients with depressive symptoms. However, it is hypothesized to work by blocking a neurotransmitter called glutamate from binding to a certain receptor in nerve cells called NMDA (N-methyl-D-aspartate). Blocking glutamate from attaching to this receptor may help regulate communication between nerve cells in the brain and improve symptoms of depression.
Note: Glutamate is also an important neurotransmitter involved in memory and learning and is important for overall healthy brain function. Some patients may experience cognitive impairments by inhibiting it, a trade-off that should be considered before starting these treatments. (TMS, in contrast, does not have this downside. Brain stimulation can improve the function of glutamate and other neurotransmitters and promote better brain function.)
Ketamine may also stimulate the production of a chemical in the brain called brain-derived neurotrophic factor (BDNF). BDNF helps the brain learn new things and adapt to new experiences. By encouraging these changes, ketamine may help change negative thought patterns that contribute to depression.
Ketamine is most commonly administered in intravenous doses ranging from 0.1 mg/kg to 0.75 mg/kg. However, these sessions are time-consuming and researchers are assessing other routes of administration, including oral, sublingual, transmucosal, intramuscular, and subcutaneous routes. For example, a phase two clinical trial is currently testing extended-release ketamine tablets.
Side Effects and Contraindications Associated With Ketamine
Ketamine may trigger side effects within a few minutes after the treatment starts. The range of side effects is somewhat unpredictable and varies greatly from patient to patient. Most side effects dissipate shortly after treatment, but some patients report symptoms that persist for several days.
Common short-term side effects include:
- Disorientation and confusion
- Loss of motor coordination
- Dizziness/lightheadedness
- Nausea
- Vomiting
- Increased blood pressure, heart rate, breathing, or body temperature
- Changes in sensory perceptions, including visual or auditory hallucinations
- Fatigue for the rest of the day after treatment
- Headache
- Tinnitus
- Temporary bruising where the needle is administered
Long-term side effects are rarer, but some patients may experience vivid dreams, hallucinations, and mania. In addition, ketamine is thought to lead to some degree of tolerance, physical dependence, and withdrawal syndrome when use stops. Ketamine withdrawal syndrome may include symptoms such as depression, excessive sleepiness, and drug cravings. Some research also suggests that long-term use may lead to neurological and cognitive problems, such as memory impairments and declines in executive functioning.
Contraindications
Ketamine should be avoided or used with extreme caution by patients:
- With a history of psychosis or schizophrenia
- With a history of excessive alcohol or substance use, as ketamine can cause euphoria and patients can become addicted to it
- Younger than 18 years old, as there are some concerns about the long-term effects of ketamine on the still-developing adolescent brain
- Who are pregnant or breastfeeding
- Older than 65 who have dementia
- With severe mobility restrictions
- With some cardiovascular conditions
- With severe liver disease
How Effective Is Ketamine?
A growing body of research supports the benefits of using ketamine to treat patients with treatment-resistant depression. Patients may start feeling the benefits just a few hours after the treatment and 50% of patients still experience improvements up to a week later. However, after six months and continued ketamine treatments, only 26% of patients were still responding and only 15% were in remission.
Ketamine is particularly known for its ability to help patients with suicidal ideation, and half of the patients who had thoughts of suicide no longer experienced them after six weeks of treatment. In addition, ketamine may also help patients replace negative beliefs with more optimistic ones, which in turn improves depression symptoms.
The main downside with ketamine is the high risk of relapse. When patients stop taking this drug, symptoms return in a matter of days or weeks, at most. However, the use of psychotherapy can prolong this symptom-free period.
How Much Does a Ketamine Infusion Cost?
Ketamine infusions start at around $400 per session. However, costs can easily add to thousands of dollars as most patients need treatments for months to manage their symptoms and avoid relapse.
Medicare and Medicaid do not currently cover ketamine IV treatments. However, some states run their own programs, so it may be worth checking the guidelines in your state regarding mental health treatment, as the guidelines are updated often. Private insurance may present similar limitations.
In most cases, insurance companies don’t cover IV ketamine as a treatment for mental health disorders. This will vary according to the insurance provider; some providers have expanded their policies to provide at least some patient treatment coverage.
Esketamine (Spravato)
In 2019, the Food and Drug Administration (FDA) approved the first new medication for major depression in decades. The drug is a nasal spray using the S-fraction from ketamine (hence the name esketamine) and sold under the commercial name Spravato. Currently, esketamine is approved to treat adults with two conditions: treatment-resistant depression and major depressive disorder with suicidal ideation or behavior.
Usually, esketamine is not seen as a first-line treatment for depression. Like ketamine, it should be considered only a part of the overall treatment. The aim is to use it in combination with antidepressant drugs or psychotherapy.
How Does Esketamine Work?
The mode of action between ketamine and esketamine is similar. The main difference is that esketamine binds more tightly to the NMDA glutamate receptors than ketamine, making it up to five times more potent. In practical terms, this means patients need a lower dose of esketamine than they do ketamine.
The nasal spray also permits a much easier application making it more accessible for patients than the IV treatments currently required to deliver ketamine. However, esketamine still needs to be administered under the supervision of a healthcare professional at a certified clinic. They will check the patient’s blood pressure and monitor for side effects for at least two hours after patients take esketamine. Ideally, patients should not drive and should avoid complex cognitive activities for a few hours after taking esketamine.
Typically, the treatment involves two phases:
- Week 1 to 4 (induction phase): During this phase, patients take esketamine twice weekly. The first dose is usually 56mg, but it may increase to 84mg in subsequent sessions. At the end of this period, patients should discuss with their doctor whether they should continue their esketamine treatment or start taking antidepressants. Patients should not stop treatment completely or they risk relapse.
- Week 5 and onward (maintenance phase): If side effects are tolerated and symptoms are improving, patients can move to a maintenance phase with weekly sessions for a while and then once every two weeks. This should still be combined with antidepressant drugs or psychotherapy to reduce the risk of relapse.
Side Effects and Contraindications Associated with Esketamine
Esketamine can cause various side effects, ranging from mild to more serious conditions. The more common side effects of esketamine are mild and include:
- Anxiety
- Dizziness and vertigo
- Feeling drunk
- Increased blood pressure
- Lethargy
- Nausea
- Numbness, often in your hands and feet
- Vomiting
- Mild allergic reaction
Most of these side effects dissipate within a few hours to a couple of days after the treatment. If they persist, patients are advised to contact their healthcare professional.
Serious side effects can include the following:
- Severe high blood pressure can lead to serious events such as seizures, stroke, or swelling inside your brain (symptoms of high blood pressure include chest pain, difficulty breathing, and headaches)
- Cognitive impairment, including difficulty concentrating and memory problems
- Urinary tract and bladder problems can cause frequent urination and pain while urinating
- Serious allergic reaction
FDA Warnings
In addition to these side effects, esketamine also comes with a Boxed Warning. This is the most serious warning from the FDA and it alerts doctors and patients about drug effects that may be exceptionally dangerous if administered incorrectly.
- Risk of sedation: Esketamine may cause excessive sleepiness and difficulty thinking clearly or, in extreme cases, loss of consciousness. This makes certain activities, such as driving, unsafe.
- Risk of dissociation: Esketamine is a dissociative drug, which means patients may feel disconnected from time, space, and even their own body or mind. It’s often described as having an “out-of-body” experience. Symptoms include vision changes, feeling hot or cold, hallucinations, numbness, ringing in the ears, and feeling time pass slower or faster than normal.
- Risk of respiratory depression: Some patients taking esketamine may experience respiratory problems, including shortness of breath and shallow breathing, often accompanied by confusion, dizziness, and nausea.
- Risk of misuse: Esketamine has a high risk of misuse. This means that it may be taken in a way other than how it’s prescribed. For this reason, esketamine is a controlled substance and doctors can only prescribe it through a drug safety program called risk evaluation and mitigation strategies (REMS).
- Risk of suicidal thoughts and behaviors: Esketamine may increase the risk of suicidal thoughts in young adults. Symptoms may include worsening depressive symptoms, sudden thoughts of suicide, and attempting suicide. These changes are more likely to occur at the beginning of the treatment or if there is a change in dosage.
Contraindications
Esketamine may not be the best option for patients with:
- Some cardiovascular conditions, including aneurysmal vascular disease or arteriovenous malformation: Esketamine increases blood pressure in the first few hours after taking the drug, which may lead to bleeding in patients with a history of aneurysms or malformations of their arteries.
- A history of brain bleeds: Increased blood pressure may also increase the risk of bleeding inside your brain and patients with a history of bleeding in the brain should not use Spravato.
- A history of psychosis: As described earlier, esketamine may cause dissociation. Patients with a history of psychosis may be at greater risk for a severe dissociative or psychotic event.
- A history of substance abuse: Esketamine is a controlled substance with a high risk of misuse and may not be the best option for patients with a history of substance abuse.
- Hypertension: Esketamine may increase blood pressure, putting increased strain on the heart, blood vessels, and brain. Some patients with high blood pressure may not be able to take esketamine.
- Liver disease. Patients with liver disease may be at greater risk of side effects from esketamine. They are also at a higher risk for longer after taking the drug.
How Effective Is Esketamine?
Before FDA approval, the efficacy of this drug was evaluated in a few clinical trials where patients received esketamine and an antidepressant medication or the antidepressant drug alone. Crucially, two of the studies failed to identify any differences between esketamine and antidepressant drugs and antidepressant drugs alone, but the third study found a small benefit with esketamine. After four weeks, almost 70% of patients were still responding to treatment but only about 30% had achieved remission.
In the long term, esketamine has reduced by half the risk of relapse by 32 weeks, but about one fourth of patients experienced symptoms again despite continuing treatment. Suicidal thinking improved considerably but there was no evidence this occurred exclusively due to esketamine.
How Much Does Esketamine Treatment Cost?
Each esketamine application costs around $400, adding up to around $800 to $1200 per week to cover two to three sessions. This may add up to anywhere between $18,000 and $45,000 per year. Esketamine may fall under your medical or pharmacy benefits depending on your coverage, so it’s important to ask your healthcare provider.
Ketamine and Esketamine May Provide Quick Results but Are Less Effective Than TMS in the Long Term
With almost 80% of patients achieving remission after a 5-day treatment, fMRI-guided theta burst stimulation is a breakthrough in neurostimulation therapy.
This protocol offers a highly effective solution for patients with treatment-resistant depression who need rapid relief but cannot commit to the six weeks of daily treatments needed for conventional TMS. As a bonus, side effects are mild and short-lived.
The durability of effect — how long response and remission last after treatment — is still unclear. However, combining it with cognitive behavioral therapy (CBT) is likely to produce strong long-term outcomes.
In contrast, ketamine and esketamine offer only a short-term solution, especially for patients with suicidal thoughts or at risk of harming themselves. As soon as patients stop their treatment, relapse can occur within a few days. As such, ketamine and esketamine should not be seen as a complete treatment, but only part of a wider plan which may include psychotherapy or even TMS to reduce the risk of relapse.
In addition, the range and severity of side effects are much greater than after TMS treatments. In fact, esketamine comes with some of the most severe FDA warnings regarding its high risk of causing dissociation, sedation, misuse, and suicidal thoughts.
| |
Ketamine
|
Esketamine
|
Accelerated fMRI-Guided iTBS
|
|
Efficacy
|
At first, 50% of patients experience significant improvements up to a week later. After six months and multiple ketamine treatments, only 26% of patients continued to respond and 15% achieved remission and were symptom-free and in remission.
|
After four weeks, almost 70% of patients continue to respond to treatment but only about 30% achieve remission.
|
About 85% of patients responded and around 78% achieved remission in a randomized controlled trial. One month after treatment, 60% of patients remained in remission.
|
|
Risk of Relapse
|
The main downside of ketamine is the high risk of relapse. When patients stop taking this drug, symptoms may return in as little as two weeks, but the use of psychotherapy can prolong the efficacy of ketamine.
|
Esketamine can reduce by half the risk of relapse by 32 weeks, but about one quarter of patients still experience symptoms again despite continuing treatment.
|
The relapse rate is estimated to be up to 50% six months after treatment discontinuation. However, maintenance sessions and/or cognitive behavioral therapy (CBT) can significantly reduce this.
|
|
Side Effects
|
Common side effects include disorientation, loss of motor coordination, dizziness, nausea and vomiting, increased blood pressure, changes in sensory perceptions, headache, tinnitus, and temporary bruising where the needle is administered.
|
Common side effects include anxiety, dizziness and vertigo, increased blood pressure, nausea and vomiting, and mild allergic reactions. Esketamine also comes with FDA warnings regarding the risk of dissociation, sedation, misuse, and suicidal thoughts.
|
Most patients experience minimal or only mild side effects, including headaches, scalp discomfort, dizziness, temporary tinnitus, and unusually high sensitivity to sound. Often, patients get used to the procedure after a few sessions, and side effects dissipate. The most serious potential side effect of TMS is a seizure, but these are extremely rare. The risk of having a seizure from a TMS session is less than 0.01% per session or less than 1 in 10,000 sessions.
|
|
Procedure
|
Ketamine is administered intravenously. Patients are closely monitored by a healthcare professional because ketamine is a dissociative drug, which means patients can feel disconnected from reality and engage in dangerous and life-threatening behaviors.
|
Esketamine is applied intranasally. Patients are closely monitored by a healthcare professional because esketamine is a dissociative drug, which means patients can feel disconnected from reality and engage in dangerous and life-threatening behaviors.
|
The procedure is non-invasive. A magnetic coil is applied over the brain, and there is no need for surgery or anesthesia. Patients can return to their normal daily activities once their daily sessions are completed.
|
|
Treatment Duration
|
Treatments may continue as long as patients experience improvements.
|
Treatment includes four weeks with applications twice each week, followed by several weeks with gradually fewer applications.
|
10 short daily sessions for one week.
|
|
Contra-indications
|
Ketamine should be avoided by patients with a history of psychosis or schizophrenia, or substance abuse. It’s also not recommended for patients younger than 18 years old, older than 65, and with some cardiovascular conditions or severe liver disease.
|
Esketamine is not recommended for patients with some cardiovascular conditions, hypertension, and liver disease, as well as a history of brain bleeds, psychosis, and substance abuse.
|
Patients with metal implants on the brain, taking certain medications, or with a history of seizures should not receive TMS treatment. In some cases, it may be possible for patients to still receive TMS even if they fit into one of the above categories. If this applies to you, discuss your suitability with your doctor.
|
|
Accessibility
|
This drug needs to be administered by a trained and qualified healthcare professional, but there are many clinics throughout the country offering this service.
|
Few clinics in the US offer accelerated fMRI-guided iTBS, so patients may have to travel to get this treatment.
|
|
Cost
|
Ketamine IV infusions start at around $400 per session. However, costs can easily add to thousands of dollars as most patients need treatments for months to manage their symptoms and avoid relapse.
|
Each esketamine application costs around $400, adding up to around $800 to $1200 per week to cover two to three sessions. This may add up to anywhere between $18,000 and $45,000 per year.
|
Accelerated fMRI-guided iTBS protocols are not currently covered by insurance. Clinics offering SAINT™ treatment charge $30,000+. At Cognitive FX, we offer accelerated fMRI-guided iTBS starting at just $9,000.
|
Cited Research











