How Much Does TMS for Depression Cost?
The type of protocol used is the most important factor influencing the price of TMS. Conventional TMS protocols are often the most affordable, while advanced protocols typically cost more, but can...

Fewer than ten locations in the United States currently offer SAINT™ treatment for individuals with treatment-resistant depression. Among the clinics that publicly share pricing, the out-of-pocket cost for the one-week program ranges from $30,000 (Kaizen) to $36,000 (Acacia).
Because SAINT is not yet covered by most insurance providers, this high price point can be a major barrier for many patients.
Fortunately, there’s an alternative that offers the same level of personalized targeting and FDA-approved theta burst stimulation—at a significantly lower cost. This approach, known as accelerated fMRI-TMS, delivers the same core elements that make SAINT so effective. The key difference lies in the software: rather than using Magnus Medical’s proprietary targeting system, Cognitive FX uses its own fMRI-based method, developed over the past decade, to identify the ideal DLPFC stimulation site.
This is the treatment offered at our clinic, Cognitive FX. Our program costs between $9,000 and $12,000, providing a more affordable option for patients seeking the benefits of SAINT. Below, we break down how our treatment compares to SAINT and what it includes.
Take our quiz to see if you’re a good fit for our accelerated fMRI-guided TMS treatment.
| Accelerated fMRI - TMS | Magnus SAINT™ TMS | |
|---|---|---|
| FDA-Approved iTBS | ✔ | ✔ |
| FDA-Approved Neuronavigators | ✔ | ✔ |
| FDA-Approved Figure 8 Coils | ✔ | ✔ |
| Number of Treatment Days | 5 | 5 |
| Treatments per Day | 10 | 10 |
| Total Treatments | 50 | 50 |
| Number of TMS Pulses | Approx. 90,000 | 90,000 |
| Resting motor threshold pulse intensity | 90–120% | 90–120% |
| FDA-Approved Personalized DLPFC Targeting | ✘ | ✔ |
| Personalized DLPFC Targeting Assists Doctor in Target Location | ✔ | ✘ |
| Personalized E Field Coil orientation | ✔ | ✘ |
| Cost | $9,000 to $12,000 | $30,000+ |
One of the most important advancements in the SAINT™ protocol is its use of functional MRI (fMRI) to guide treatment. Rather than using standard anatomical landmarks or trial-and-error targeting, both SAINT and our approach use fMRI scans to identify the precise region of the dorsolateral prefrontal cortex (DLPFC) that is functionally connected to the subgenual anterior cingulate cortex (sgACC)—a brain region involved in mood regulation. This individualized targeting improves treatment precision and clinical outcomes.
After identifying the ideal stimulation site, both protocols use advanced neuronavigation systems approved by the FDA to ensure the magnetic coil is accurately positioned over the target area during every session. This real-time guidance system ensures consistency and effectiveness across all treatments.
Instead of standard repetitive transcranial magnetic stimulation (rTMS), both protocols use intermittent theta-burst stimulation (iTBS)—a newer form of FDA-approved brain stimulation. iTBS is delivered in rapid bursts, allowing for shorter treatment times while achieving similar or greater clinical effects compared to traditional TMS. It also enables the delivery of multiple sessions per day without diminishing the treatment’s impact.
The SAINT protocol delivers ten sessions of iTBS per day, spaced 50 minutes apart, over five consecutive days. Our treatment follows this same schedule, providing the same level of treatment intensity and cumulative stimulation dosage. This accelerated schedule is a key component of the protocol’s rapid and sustained antidepressant effects.
In contrast to the four to six weeks it takes to receive rTMS, it is also much more practical for patients to complete these protocols amidst work and life commitments.
Clinical studies on SAINT have demonstrated response and remission rates that are significantly higher than those seen with standard TMS or antidepressant medication. Similarly, our patients report substantial symptom relief, particularly from those who have not responded to other forms of treatment. While we do not have published clinical trials under the SAINT name, our approach mirrors its core methodology and delivers similarly promising outcomes.
Further reading: Alternative treatments for major depression: TMS, ECT, Ketamine & more
While our treatment protocol at Cognitive FX mirrors the SAINT protocol in almost every meaningful way, there are three primary differences that patients should be aware of.
SAINT treatment uses proprietary software developed by Magnus Medical to analyze fMRI data and pinpoint the ideal stimulation site in the dorsolateral prefrontal cortex. This software uses a specific algorithm to identify the region most anti-correlated with the subgenual anterior cingulate cortex (sgACC), which is believed to drive depression symptoms.
At Cognitive FX, we also use fMRI scans to determine the optimal stimulation site, but our approach relies on clinical expertise rather than Magnus Medical’s proprietary algorithm. A trained neuroscientist and physician use advanced post-processing of each patient’s brain to identify the ideal target based on functional connectivity patterns. While the methodology differs slightly, the end result—precision targeting of the DLPFC using fMRI—is functionally the same.
This distinction is similar to the difference between a brand-name medication and a compounded version: same active components and clinical intent, just without the proprietary branding. Cognitive FX has been providing clinical fMRI scans for over 10 years and has developed many of the tools needed to process scans in-house, avoiding expensive licensing fees. This allows us to provide the individualized target location at a lower cost.
Another key difference is the cost. SAINT treatment is currently priced between $30,000 and $36,000 for a one-week course, depending on the clinic. These costs are not covered by insurance and must be paid out of pocket. The only exception is Medicaid and Medicare patients who receive SAINT in hospital settings (more on this below).
In contrast, our treatment at Cognitive FX ranges from $9,000 to $12,000, offering the same protocol structure and therapeutic benefits at a much more accessible price point. This affordability allows more patients—especially those who don’t qualify for insurance coverage or who are managing financial constraints—to access cutting-edge treatment for treatment-resistant depression.
At Cognitive FX, we use E-Field modeling of your brain to ensure the dosage is accurate. Currently, Magnus Medical does not use the E-Field.
For each patient, the fMRI data ensures that we place the coil correctly on the scalp, while the E-Field model ensures that we target the magnetic field correctly for your individualized brain.
Our clinic, Cognitive FX, based in Provo, Utah, is proud to be among the first in the nation to offer accelerated fMRI-guided TMS for treatment-resistant depression.
Over the last two decades, the leaders of our clinic have pioneered treatment for patients experiencing lingering symptoms from mild traumatic brain injury. Now, we’re bringing our team’s expertise in functional magnetic resonance imaging (fMRI) and clinical psychology to help patients recover from moderate to severe depression.
Our TMS treatment is ideal for most patients with treatment-resistant depression, but we have specific eligibility criteria for safety:
Currently, our standard treatment starts at $9,000 and includes:
Our upgraded plan, priced at $12,000, includes additional services such as:
If you are interested in receiving fMRI-guided accelerated TMS therapy at Cognitive FX, see if you’re a good fit for treatment.
SAINT TMS, which stands for Stanford Accelerated Intelligent Neuromodulation Therapy, is an FDA-cleared form of transcranial magnetic stimulation (TMS) developed by Nolan Williams at Stanford University. Like traditional TMS, the treatment uses magnetic pulses to stimulate neurons in a specific area of the brain, the dorsolateral prefrontal cortex (DLPFC), which is consistently affected by depression and other mental health disorders.
However, SAINT TMS uses intermittent theta-burst stimulation (iTBS), as well as fMRI and neuronavigation for precision targeting of the DLPFC. It is also delivered in a condensed treatment timeframe of just five days.
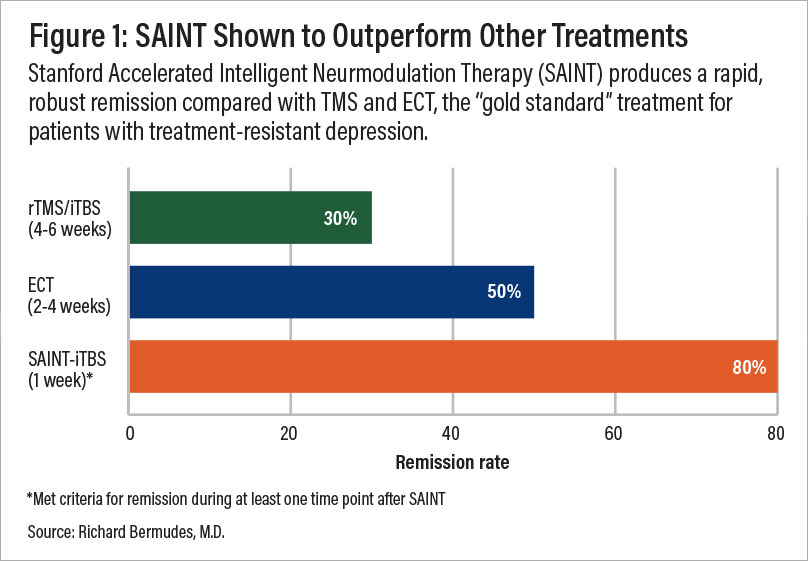
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
SAINT TMS is considered the "gold standard" treatment for treatment-resistant depression, a type of depression that has not responded to multiple rounds of antidepressant medication and psychotherapy.
Clinical trials that show SAINT TMS to be highly effective include:
High response rate: In a double-blind, randomized controlled trial, about 85.7% of patients responded to SAINT-iTBS treatment. This means they met pre-defined criteria for a reduction in their depressive symptoms.
High remission rate: Around 78.6% of patients in the same trial met the criteria for remission, indicating a significant decrease or disappearance of depression signs and symptoms.
Rapid relief: These results were achieved in a single week of treatment, making it one of the fastest-acting depression treatment options available. A month after treatment, 60% of the trial participants were still in remission.
SAINT TMS is generally considered a safe procedure. Side effects are typically mild, short-lived, and less severe than those associated with antidepressant medications.
Common side effects: The most commonly reported side effects are headaches, scalp discomfort, and muscle twitching in the face or scalp during treatment. These usually disappear after the first few sessions.
Rare side effect: Seizures are a potential side effect of TMS but are extremely rare, occurring in less than 0.01% of sessions (less than 1 in 10,000). SAINT TMS further reduces the risk of seizures by applying pulses at a lower intensity (80% of the brain's motor threshold) compared to rTMS.
While SAINT TMS can be highly effective, it may not be suitable for everyone. Here are some factors that may indicate you are a good candidate for SAINT TMS:
Treatment-resistant depression: If you have been diagnosed with major depressive disorder (MDD), particularly treatment-resistant depression, and other treatment options like medication and therapy haven't provided relief, SAINT TMS may be a viable option.
Antidepressant side effects: If you are experiencing intolerable side effects from antidepressant medications, SAINT TMS could be a good alternative.
Desire for fast results: SAINT TMS offers a relatively quick treatment duration of one week, making it suitable for individuals seeking faster relief from depressive symptoms.
Limited time for treatment: If your work or personal commitments make it difficult to adhere to the longer treatment schedules of traditional TMS protocols, SAINT TMS's condensed time frame might be more manageable.
Factors that may indicate you’re not a good candidate include:
Metal implants: Individuals with metal implants or objects in their heads close to the treatment area cannot receive TMS. This is because the magnetic pulses could interfere with the implanted devices.
History of seizures: Patients with a history of seizures are typically not suitable candidates for TMS, including SAINT TMS.
It's crucial to consult with a healthcare professional to determine if SAINT TMS is right for you. They can assess your medical history, current condition, and individual needs to provide personalized recommendations.
Currently, SAINT treatment is not covered by private insurance providers. While healthcare providers can prescribe transcranial magnetic stimulation (TMS) for depression, only traditional repetitive TMS (rTMS) is typically eligible for coverage. Each insurer has its own criteria—some require patients to have failed multiple antidepressant medications and psychotherapy, and may specify medication types or symptom severity to qualify.
Even with approval, patients are usually responsible for copays and meeting deductibles. Before starting treatment, it’s important to confirm how much of your deductible and out-of-pocket maximum has been met. For those needing help with expenses, payment plans, medical financing, and credit options like CareCredit may be available.
For patients paying out of pocket, there are no restrictions on using more advanced TMS approaches. Clinics across the U.S. offer newer protocols, such as our accelerated fMRI-TMS protocol, that deliver improved outcomes with mild side effects—though these options come at a higher cost.
Access to SAINT treatment is expected to expand starting in 2025. The Centers for Medicare and Medicaid Services (CMS) have announced that hospitals will be reimbursed $19,703 for the full SAINT protocol, beginning July 1, 2025. New billing codes include daily reimbursement of $3,750.50 for SNT therapy and $950 for targeting, supporting wider availability of this promising treatment for patients with treatment-resistant depression.
Take our personalized TMS treatment quiz here. The form will guide you through a short series of questions to see if you’re a good fit to receive treatment with us.
DISCLAIMER: SAINT™ is a trademark of The Board of Trustees of the Leland Stanford Junior University (“Stanford”) and has exclusively licensed such mark to Magnus Medical. Cognitive FX is neither endorsed by Stanford nor utilizes Magnus Medical equipment nor claims to be offering the SAINT™ protocol as prescribed by Stanford University et. al. or Magnus Medical. We provide fMRI-guided intermittent theta burst TMS with target locations determined by fMRI and our prescribing physician.

Dr. Mark D. Allen holds a Ph.D. in Cognitive Science from Johns Hopkins University and received post-doctoral training in Cognitive Neuroscience and Functional Neuroimaging at the University of Washington. As a co-founder of Cognitive Fx, he played a pivotal role in establishing the unique and exceptional treatment approach. Dr. Allen is renowned for his pioneering work in adapting fMRI for clinical use. His contributions encompass neuroimaging biomarkers development for post-concussion diagnosis and innovative research into the pathophysiology of chronic post-concussion symptoms. He's conducted over 10,000 individualized fMRI patient assessments and crafted a high-intensity interval training program for neuronal and cerebrovascular recovery. Dr. Allen has also co-engineered a machine learning-based neuroanatomical discovery tool and advanced fMRI analysis techniques, ensuring more reliable analysis for concussion patients.

The type of protocol used is the most important factor influencing the price of TMS. Conventional TMS protocols are often the most affordable, while advanced protocols typically cost more, but can...
Transcranial magnetic stimulation (TMS) has been FDA-approved for treating major depressive disorder (MDD) since 2008 and is a well-established treatment option, especially for patients who haven’t...

Transcranial magnetic stimulation (TMS) and neurofeedback are gaining popularity as non-invasive, medication-free options for treating depression—especially for people who haven’t found relief from ...

Access to SAINT™ TMS in the U.S. remains extremely limited, with fewer than ten clinics currently offering the protocol. On top of that, the cost—often exceeding $30,000 and rarely covered by...

If you’re considering Transcranial Magnetic Stimulation (TMS) — a procedure that uses electromagnetic pulses to improve depression symptoms — you may be wondering what the treatment actually feels...

Patients considering transcranial magnetic stimulation (TMS) often wonder whether or not they can continue taking their antidepressant medication while undergoing TMS treatment.