TMS vs. tDCS for Depression: Which Is Better?
For many people living with major depression, antidepressant medications either don’t work or only get them part of the way to recovery. Symptoms may ease for a while, but then return, or never fully...

If you’re considering brain stimulation therapy for treating major depression, you may wish to understand the differences between repetitive transcranial magnetic stimulation (rTMS) and deep transcranial magnetic stimulation (dTMS).
This article outlines the key differences and similarities between the two treatments to help patients decide which type is best for them.
It also discusses the key limitations of these protocols and explains how Stanford Intelligent Accelerated Neuromodulation Therapy (SAINT-iTBS) solves them to provide even more effective treatment outcomes.
Specifically, we cover:
Repetitive TMS (rTMS) was the first transcranial magnetic stimulation protocol approved by the FDA in 2008.
Like all types of TMS therapy, this non-invasive treatment uses electromagnetic pulses to stimulate nerve cells in areas of the brain that are associated with depression and other mental health conditions. By restoring normal activity levels, TMS can lead to relief in depression symptoms that persist long after the end of treatment.
The success of rTMS has led to developing other forms of TMS, including deep TMS (dTMS).
rTMS targets an area of the brain called the dorsolateral prefrontal cortex (DLPFC).
This region is one of the most consistently affected by depression. Low activity is especially pronounced in the left dorsolateral prefrontal cortex, which is responsible for positive feelings, including feeling rewarded for certain behaviors.
During rTMS treatment, a figure-8-shaped electromagnetic coil is placed directly over the DLPFC to stimulate and help restore normal activity levels in that area. This results in decreased depressive symptoms for many patients.
In contrast, deep TMS relies on a magnetic coil called the Hesed coil (or H-coil for short) to deliver deeper and broader penetration of electromagnetic stimulation into the brain.
Patented in 2002 by the NIH, the coil looks like a motorcycle helmet. After some simple measurements, operators place the helmet on the patient’s head to find the coil location.
Over the years, the Hesed coil has been developed to stimulate different brain structures and is now used in 3 different models: H-7, H-4, and H-1 coils. The H-coil can reach about 3-4 cm depth of penetration during treatment compared to only 0.5-0.7 cm with the standard figure-8 coil.
Because deep TMS treatment involves broader and deeper stimulation compared to the highly targeted approach of rTMS, there is a greater potential for confounding variables that may have unintended effects on brain function.
Inevitably, this increases the risk of serious side effects. For example, one study comparing the two methods found a low seizure rate of 0.14 per 1000 patients after rTMS, compared to a much higher 5.6 per 1000 patients after dTMS.
Despite having slightly higher risks and potential for confounding variables, dTMS still has a higher efficacy compared to rTMS.
Repetitive TMS improves symptoms in about 50% of patients with depression, with over 30% achieving remission. Patients who have not responded to multiple antidepressant medications are more challenging to treat, but around 30% still respond to treatment, and 19% achieve remission.
In comparison, after 30 sessions of dTMS, 82% of patients responded and 65% achieved remission. Even just 20 sessions led to a 74% response and 58% remission rate. Similar to rTMS, dTMS is less effective with patients who had previously failed to improve after multiple antidepressant medications, but almost 30% still reached remission.
This trend was confirmed in a study directly comparing rTMS and dTMS. The values were overall slightly lower than in other studies, but still show about 40% of patients achieved remission after rTMS, compared to over 60% with dTMS.
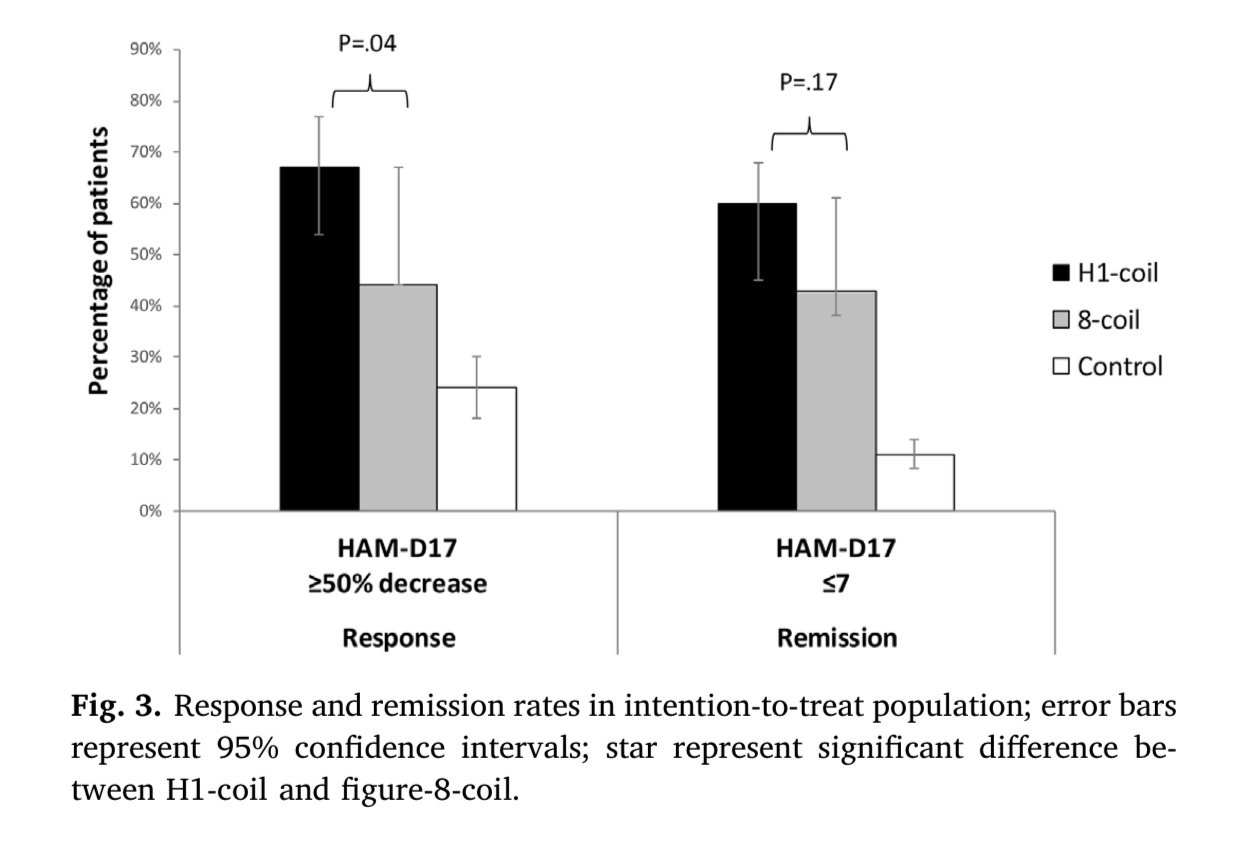
rTMS has become a well-accepted treatment for depression and there are many clinics around the country offering this service.
In contrast, far fewer clinics offer dTMS, and patients may have to travel long distances to find a location offering this treatment.
Although rTMS and dTMS follow different protocols and use different equipment, there are still some similarities, including:
Duration of treatment: Both treatments involve 4-6 weeks of daily treatment sessions 5 days per week. Deep TMS sessions are slightly shorter at 20 minutes, while rTMS lasts about 37 minutes.
Suitability: Both rTMS and dTMS are suitable for most patients with depression, excluding patients with a ferromagnetic implant located in or around the area where the coil needs to be placed, such as cochlear implants, medication pumps, aneurysm clips or coils, stents, or even bullet fragments. Patients with these implants cannot undergo any type of TMS because the magnetic fields could malfunction the implanted devices. Braces and dental fillings are acceptable for treatment.
Mild side effects: Side effects after rTMS or dTMS are typically mild and short-lived. For both treatments, common side effects include headaches, scalp discomfort, and dizziness. These side effects tend to subside shortly after treatment.
Costs: Costs are similar between both treatments. Typically, TMS sessions cost about $250-$300, which adds up to a total cost between $6,000 and $12,000 for the complete treatment.
Insurance: Both rTMS and dTMS are currently covered by many insurance providers. Coverage policies can vary depending on the insurer, so it's always best to check with your insurance company to confirm their specific TMS coverage.
FDA approval status: Both rTMS and dTMS are FDA-approved for treating depression. The deep TMS H-1 model was approved in 2013 for the treatment of major depressive disorder (MDD) and treatment-resistant depression (TRD), and the H-7 model was approved in 2018 for the treatment of obsessive-compulsive disorder (OCD). In 2020, dTMS also became the first system approved to help adults quit smoking. Finally, in 2021, the use of dTMS was further expanded to include the treatment of depressive episodes and accompanying anxiety symptoms, receiving FDA approval to treat anxious depression.
rTMS and dTMS are both safe and worthwhile depression treatment options to consider, especially with their effectiveness rates and mild side effects compared to most other treatments.
With that said, patients should be aware of the two main limitations of these treatments.
As discussed above, standard rTMS and dTMS protocols are typically administered with daily sessions, 5 days per week over 4 to 6 weeks. This lengthy and demanding treatment schedule is often difficult for patients to complete amidst work, childcare, and other life commitments.
It’s also a longer-than-ideal time for patients experiencing severe depression symptoms.
In the standard rTMS protocol, coil placement is typically determined using the "5 cm method," which involves manually measuring distances between the patient’s nose, ears, and the top of the head. However, this approach can be imprecise due to variations in head size, shape, and individual brain structure, which may significantly affect treatment outcomes. Even a miss of the left DLPFC by a few millimeters can reduce the effectiveness of the treatment.
In some cases, an EEG cap is used to help locate the treatment area. While research suggests this method is somewhat more accurate and reliable than the 5 cm method, the difference is minor and not likely to result in substantially improved outcomes. Additionally, factors like a simple haircut can affect how the cap fits, potentially causing variations between sessions.
As discussed above, the dTMS protocol applies broader targeting, leading to a higher risk of side effects and the potential for confounding variables to affect brain function in unintended ways.
Stanford Intelligent Accelerated Neuromodulation Therapy (SAINT™), which was FDA-approved to treat major depressive disorder (MDD) in 2022, addresses both of these issues.
First, it uses functional MRI to map brain connectivity and pinpoint the treatment target area for each patient, and neuronavigation to position the magnetic coil directly over the L-DLPFC for every treatment session.
Second, the SAINT protocol uses an accelerated form of TMS called intermittent theta burst stimulation (iTBS). This condenses treatment into a single week, making it much more convenient for patients to fit into their lives, and offering faster relief. Patients sit for 10 short sessions each day, for 5 days, with a 50-minute break between sessions.
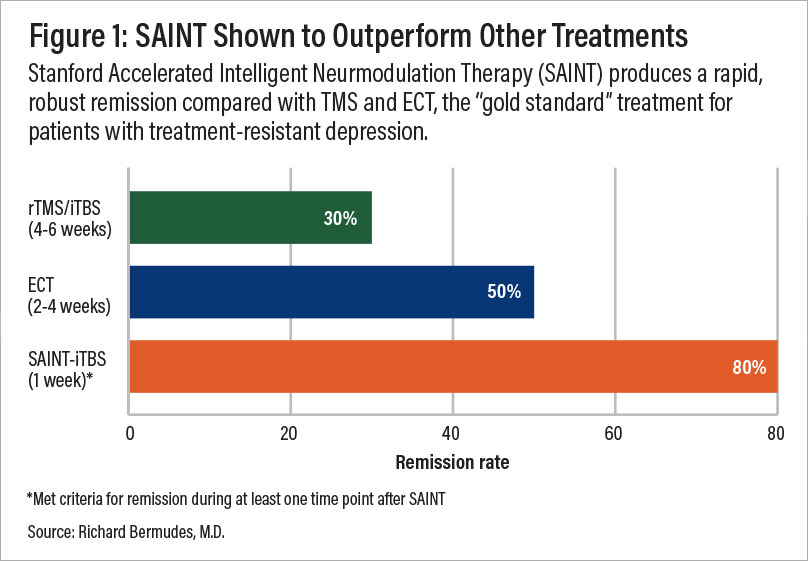
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
Following this protocol significantly reduced depressive symptoms and suicidal ideation within 5 days, without negative side effects. In a double-blind randomized controlled trial, about 86% of patients responded to the treatment (meaning they met prespecified criteria for reduced depressive symptoms) and around 79% met the remission criterion. All the individuals in the study had treatment-resistant depression and had failed at least two other depression treatments. One month after the clinical trial, 60% were still in remission. (Find the full text of the sham-controlled study here.)
The speed with which SAINT treatment can achieve high response and remission rates, combined with minimal side effects, makes it one of the best fast-acting depression treatments available today.
With that said, there are some practical barriers that make SAINT treatment less accessible:
Our team at Cognitive FX, based in Provo, Utah, provides an alternative to SAINT TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
| Accelerated fMRI - TMS | Magnus SAINT™ TMS | |
|---|---|---|
| FDA-Approved iTBS | ✔ | ✔ |
| FDA-Approved Neuronavigators | ✔ | ✔ |
| FDA-Approved Figure 8 Coils | ✔ | ✔ |
| Number of Treatment Days | 5 | 5 |
| Treatments per Day | 10 | 10 |
| Total Treatments | 50 | 50 |
| Number of TMS Pulses | Approx. 90,000 | 90,000 |
| Resting motor threshold pulse intensity | 90–120% | 90–120% |
| FDA-Approved Personalized DLPFC Targeting | ✘ | ✔ |
| Personalized DLPFC Targeting Assists Doctor in Target Location | ✔ | ✘ |
| Personalized E Field Coil orientation | ✔ | ✘ |
| Cost | $9,000 to $12,000 | $30,000+ |
This protocol of TMS is:
To improve outcomes for our patients, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.
|
dTMS |
rTMS |
Accelerated fMRI iTBS |
|
|
Stimulation |
dTMS is a brain stimulation technique that uses an H-coil to target deeper brain structures. |
rTMS is a brain stimulation technique that uses a figure-8 magnetic coil to deliver magnetic pulses. |
Accelerated fMRI-iTBS is a brain stimulation technique that uses a figure-8 magnetic coil to deliver magnetic pulses. |
|
Depth of Stimulation |
dTMS reaches about 3–4 cm deep into the brain, targeting multiple subcortical regions. |
rTMS reaches superficial areas in the brain (about 0.5–0.7 cm). |
Accelerated fMRI-iTBS reaches superficial areas in the brain (about 0.5–0.7 cm). |
|
Target |
dTMS can target multiple regions of the brain depending on what is being used to treat. |
rTMS targets a small area in the brain called the dorsolateral prefrontal cortex (DLPFC). |
Accelerated fMRI-iTBS targets a small area in the brain called the dorsolateral prefrontal cortex (DLPFC). |
|
Treatment Duration |
Sessions typically last 20 minutes. |
Sessions last 37 minutes. |
Sessions last 10 minutes. |
|
Frequency of Sessions |
Frequency varies; patients typically receive daily sessions (5 days per week for 4–6 weeks). |
Patients receive daily sessions 5 days per week for 4–6 weeks. |
Patients receive 10 short sessions daily for 5 days. |
|
Side |
Side effects are mild, short-lived, and may include headaches, scalp discomfort & dizziness. Slightly higher risk for seizures than rTMS. |
Side effects are mild, short-lived, and may include headaches, scalp discomfort & dizziness. |
Side effects are mild, short-lived, and may include headaches, scalp discomfort & dizziness. |
|
Efficacy |
About 80% of patients responded and 65% achieved remission after 30 dTMS sessions. |
Around 50% of patients respond to treatment and 30% reach remission. |
Around 86% of patients respond to treatment and 79% reach remission. |
|
FDA |
FDA-approved for treating depression, OCD, and smoking cessation. |
FDA-approved for treating depression, migraines, and some other neurological conditions. |
FDA-approved to treat depression. |
|
Accessibility |
Availability is limited due to the specialized equipment and expertise required. |
More widely available in TMS clinics and treatment centers. |
Availability is limited due to the specialized equipment and expertise required. |
|
Cost |
Can be a costly treatment. Sessions cost about $250–$300, for a total $6,000–$12,000 for the complete treatment. |
Can be a costly treatment. Sessions cost about $250–$300, for a total $6,000–$12,000 for the complete treatment. |
Can be a costly treatment. Treatment may cost $6,000 to $15,000. |
|
Insurance |
Treatment is covered by some insurance companies. |
Treatment is covered by some insurance companies. |
Treatment isn't currently covered by insurance. |

Dr. Mark D. Allen holds a Ph.D. in Cognitive Science from Johns Hopkins University and received post-doctoral training in Cognitive Neuroscience and Functional Neuroimaging at the University of Washington. As a co-founder of Cognitive Fx, he played a pivotal role in establishing the unique and exceptional treatment approach. Dr. Allen is renowned for his pioneering work in adapting fMRI for clinical use. His contributions encompass neuroimaging biomarkers development for post-concussion diagnosis and innovative research into the pathophysiology of chronic post-concussion symptoms. He's conducted over 10,000 individualized fMRI patient assessments and crafted a high-intensity interval training program for neuronal and cerebrovascular recovery. Dr. Allen has also co-engineered a machine learning-based neuroanatomical discovery tool and advanced fMRI analysis techniques, ensuring more reliable analysis for concussion patients.

For many people living with major depression, antidepressant medications either don’t work or only get them part of the way to recovery. Symptoms may ease for a while, but then return, or never fully...

A major challenge for patients with depression is that traditional antidepressant medications often take weeks or months to show results — if they work at all. This delay can be especially...

Transcranial magnetic stimulation (TMS) and neurofeedback are gaining popularity as non-invasive, medication-free options for treating depression—especially for people who haven’t found relief from ...
Transcranial magnetic stimulation (TMS) has been FDA-approved for treating major depressive disorder (MDD) since 2008 and is a well-established treatment option, especially for patients who haven’t...
Clinics across the U.S. now offer TMS therapy specifically for anxiety. While early research suggests TMS may be an effective treatment, only a handful of studies have focused exclusively on anxiety.

With the original form of TMS therapy for major depressive disorder—known as repetitive transcranial magnetic stimulation (rTMS)—some patients begin noticing improvements within the first week of...