Traditional antidepressant medications involve a trial period of weeks or months before it can be determined whether or not they are working. If a medication doesn’t work — and it’s been shown that traditional antidepressant medications only work for about one-third of patients — then the patient must begin another trial with a different drug and go through the process all over again.
Throughout these trials, patients often experience unpleasant side effects that impact their quality of life. And beyond a second failed antidepressant drug, data suggests that less than 2% of patients have success with a third or fourth medication.
Patients need new treatment options that help relieve their symptoms faster, ideally with fewer side effects. In this article, we discuss what’s available at the moment, including:
Transcranial Magnetic Stimulation (TMS)
TMS is a noninvasive, FDA-approved procedure that uses magnetic pulses to restore normal activity levels in a part of the brain called the dorsolateral prefrontal cortex (DLPFC) which is consistently affected by depression and other mood disorders. During a TMS session, an electromagnetic coil is placed over the patient’s head directly above the DLPFC, and pulses are applied at specific intervals to stimulate nerve cells in that area.
Patients who receive newer forms of TMS can experience relief from their depression in a single week of treatment. TMS also has mild side effects compared to antidepressant medications which we’ll discuss below.
Types of TMS
Repetitive Transcranial Magnetic Stimulation (the Original Method of TMS)
The original form of TMS was first used in the treatment of depression in the mid-90s. This procedure — called repetitive transcranial magnetic stimulation (rTMS) — involves the application of repeated electromagnetic pulses delivered by a magnetic coil placed on the patient’s scalp over the DLPFC area.
Usually, patients receive 20-30 TMS sessions, delivered daily over four to six weeks, with each session lasting up to 40 minutes. Many patients begin feeling better after just two weeks. About 50% of patients respond well to treatment with over 30% showing complete remission. When combined with psychotherapy, success rates are even more impressive with response and remission rates of ~66% and ~55% respectively.
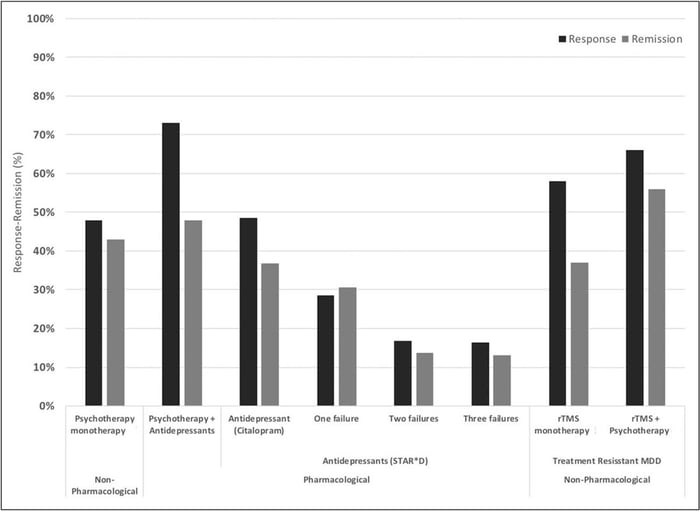
Response and remission rates of various monotherapeutic and combinatory antidepressant treatments based on the largest studies and datasets available. [Source]
Accelerated TMS (Reduces Treatment Time to a Single Week)
Showing up to daily sessions for four to six weeks has been impractical for many patients due to work and life commitments. To solve this issue, researchers developed accelerated TMS, where patients undergo multiple TMS sessions per day and can complete treatment in a single week.
Accelerated TMS is as safe as daily rTMS and potentially more effective. In addition, patients receiving multiple sessions each day seem to experience improvements in symptoms faster than those receiving daily sessions, and the effects are still visible months after their treatment.
Intermittent Theta-Burst Stimulation (a New Method of TMS)
In 2018, the FDA approved a new version of accelerated TMS called Intermittent Theta-Burst Stimulation (iTBS). Theta waves are the frequencies the hippocampus uses to connect to other brain circuits and are utilized in memory formation, meditation, and certain phases of sleep. Theta burst stimulation uses a different magnetic pulse (in triplets) and delivers the treatment in three minutes (compared to 37 minutes in rTMS).
This method can produce rapid reductions in depression symptoms with many patients experiencing improvements shortly after starting their treatment. In addition, patients also noticed a decrease in suicidal ideation, suggesting that iTBS could be an option to rapidly treat patients at high risk of suicide.
fMRI-Guided iTBS (the Newest and Most Effective Method of TMS)
In the standard rTMS and iTBS protocols, the location to apply the coil is calculated based on taking manual measurements between the patient’s nose, ears, and top of the head. However, due to variations in head size and shape, or differences in brain organization between patients, studies have found this method of coil placement can be imprecise and lead to less consistent outcomes. Even missing the DLPFC by a few millimeters is enough to produce poorer results.
To overcome this limitation, researchers began using functional MRI and neuronavigation devices to determine exactly where the DLPFC is located in the depressed patient’s brain. This method, developed by a team from Stanford University, is known as the SAINT™ protocol. SAINT stands for Stanford Intelligent Accelerated Neuromodulation Therapy. The FDA approved SAINT depression treatment in September 2022, and it is now regarded as the “gold standard” treatment for treatment-resistant depression.

A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
The SAINT™ protocol involves 10 sessions per day over five days for 50 sessions total. In a double-blind randomized clinical trial, about 85.7% of patients responded to the SAINT-iTBS treatment (meaning they met prespecified criteria for reduced depressive symptoms) and around 78.6% met the remission criterion. All the individuals in the study had treatment-resistant depression and had failed at least two other depression treatments. One month after treatment, 60% were still in remission.
The speed with which SAINT-iTBS can achieve high response and remission rates makes it one of the best rapid-acting treatments for depression available today. However, there is limited data on the durability of these outcomes and how well they hold up over time. Some patients see lasting remission or symptom reduction, while others need follow-up treatments to maintain the effectiveness. Studies show that TMS maintenance sessions can be extremely helpful, sometimes allowing patients to stay symptom-free for up to eight years.
In addition, combining TMS with cognitive behavioral therapy (CBT), as we do at our clinic, is likely to produce the best long-term outcomes for patients. While there are no studies yet combining iTBS and CBT, one study found that combining rTMS and CBT nearly doubled remission rates for patients.
Side Effects and Contraindications
TMS is a safe procedure with only mild and short-lived side effects, such as headaches, neck pain, or a tingling sensation in the scalp. These symptoms usually go away after a few sessions. The most serious complication is a seizure, but the risk is extremely low — less than 0.001% per session. Even if a seizure does occur, patients recover quickly and fully without any long-term effects.
TMS cannot be used if you have certain metallic implants or objects in your brain near where the coil is placed. This includes devices like cochlear implants, Internal Pulse Generators, medication pumps, aneurysm clips or coils, stents, or bullet fragments. However, braces and dental fillings are safe and won't interfere with treatment.
Some medications can increase the risk of seizures and might prevent you from having TMS. If you are on medication, it’s important to talk to your healthcare professional before starting TMS. For patients taking drugs with a high risk of seizures, TMS should be used with caution.
Receiving fMRI-Guided TMS at Cognitive FX
Our clinic in Provo, Utah, provides an alternative to SAINT™ TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT™ (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
This accelerated protocol of iTBS is:
- Safe: Widely tolerated and associated with mild, short-lasting side effects.
- Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
- Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work commitments (compared to 4 to 6 weeks of standard TMS and accelerated TMS protocols).
To improve patient outcomes, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.
Other Fast-Acting Depression Medications for Treatment-Resistant Depression
Recent efforts to develop new pharmaceuticals for treating depression have primarily focused on increasing the levels of serotonin in the brain. However, the idea that depression is caused by low serotonin levels is still just a hypothesis.
Over the past three decades, compelling evidence has indicated that the neurotransmitter glutamate also plays a role in depressive symptoms. This research has enhanced our understanding of the brain mechanisms behind depression and has led to the development of new fast-acting antidepressants, such as ketamine, esketamine, and dextromethorphan-bupropion.
These antidepressants bind to N-methyl-D-aspartate (NMDA) receptors in the brain and block the activity of glutamate, an excess of which is thought to contribute to depression. However, glutamate is also an important neurotransmitter involved in memory and learning and is important for overall healthy brain function. By inhibiting it, some patients may experience cognitive impairments, a trade-off that patients should consider before starting any of these treatments. (TMS, in contrast, does not have this downside. Brain stimulation can improve the function of glutamate and other neurotransmitters and promote better brain function.)
Ketamine
Ketamine was approved by the FDA in 1970 to be used as an anesthetic. In 2000, a clinical trial showed that ketamine could be used to improve the mood of patients living with depression. Since then, multiple clinical trials have shown that ketamine is extremely fast-acting compared with traditional antidepressants and can relieve depressive symptoms, including suicidal ideation, for a period that can last days or weeks. Around 60% of patients experienced the benefits of ketamine up to three days after a single treatment and about 40% of these patients still had not relapsed a month later. However, after six months and multiple ketamine treatments, only 26% of patients were still responding and only 15% were symptom-free and in remission.
In chemical terms, ketamine is a chiral molecule, meaning it has two chemical forms that are mirror images of each other: R-Ketamine and S-Ketamine. Racemic ketamine is a mixture of both R-Ketamine and S-Ketamine in equal parts and is the most commonly used form of ketamine in clinical settings.
Ketamine is administered to patients intravenously. During this procedure, patients are closely monitored by a healthcare professional because ketamine is a dissociative drug, which means patients can feel disconnected from reality and engage in dangerous and life-threatening behaviors. In addition, patients may also experience severe side effects, including:
- Dissociation
- Intoxication
- Sedation
- High blood pressure
- Dizziness
- Headache
- Blurred vision
- Anxiety
- Nausea and vomiting
A typical treatment plan includes four to six sessions over two to three weeks. Some patients need booster sessions, typically once a month or once every three months, depending on the patient. To boost the effects of ketamine, it is recommended to combine it with a form of psychotherapy, such as cognitive behavioral therapy (CBT).
The surge in demand for this drug has paved the way for a cottage industry of clinics offering ketamine treatments. Some companies make sweeping claims that they’re able to treat dozens of conditions, including depression, anxiety, chronic pain, PTSD, bipolar disorder, and migraines. Such claims are currently not backed by scientific studies. As the industry is unregulated, the quality of care received at these clinics can be highly variable. Many clinics are not run by doctors of psychiatry and are simply pain management clinics that have added ketamine to their list of treatments or are spas with staff not trained in mental health treatment. In addition, it can be expensive. Typically, treatments cost between $400 to $1,000 per session, which can add up to thousands for the full treatment. If you’re considering this option, our advice is to discuss it with your family doctor before committing to any clinic.
Esketamine (Brand Name: Spravato)
Ketamine shows some promise in treating treatment-resistant depression, but the IV administration is time-consuming. To overcome these issues, researchers developed a new antidepressant using only the S-ketamine fraction, aptly named Esketamine. In 2019, the FDA approved this product as a nasal spray for easy application, sold under the brand name Spravato.
Clinical studies have shown that S-ketamine has fast antidepressant effects, with some patients reporting a reduction in symptoms within hours of administration. This is in contrast to traditional antidepressant medications that can take weeks or months to take effect. This makes it particularly beneficial for patients who have not responded to other treatments or are in urgent need of treatment due to severe suicidal tendencies. After an eight-week treatment, about one third of patients had achieved remission and, of these, about one third had not relapsed more than eight months later.
Spravato comes with specific guidelines for administration due to potential side effects such as nausea, headaches, dizziness, disorientation, and dissociation immediately after use. It must be administered at certified treatment centers under the supervision of a mental health care professional. Ideally, esketamine should be used in conjunction with a conventional antidepressant medication. The aim is to use esketamine for fast relief from depression symptoms until the other medication takes effect.
In most cases, patients receive esketamine twice a week for the first month, and then if patients improve, they continue to receive the drug at a lower frequency for a few weeks or months. Typically, this means treatments start as weekly, then drop to every two weeks, then monthly, etc.
Arketamine
Researchers have also tested the R-ketamine fraction — called arketamine — to treat major depression and other mental health conditions, but this drug has not received approval from the FDA yet.
Preliminary results are encouraging, with antidepressant effects faster and longer than ketamine and fewer side effects, especially in terms of dissociation and hallucinations. Clinical trials are currently underway in the US and other locations around the world.
In addition to its antidepressant-like effects, arketamine may help treat patients with various neurological disorders, such as Parkinson's disease and multiple sclerosis. Moreover, arketamine has anti-inflammatory, bronchodilating, and neuroprotective effects, which means it could be a new drug for patients with neurological disorders and inflammatory diseases.
Dextromethorphan-bupropion (Brand Name: Auvelity)
A new fast-acting medication called Auvelity was just approved by the US Food and Drug Administration to treat patients with major depressive disorder (MDD). Auvelity is a combination of two already existing drugs: dextromethorphan and bupropion.
Dextromethorphan is commonly used in cough syrups to provide temporary relief from cough. Recent research showed it can also be used to alleviate depressive symptoms, but it’s metabolized very quickly in the body. Bupropion is an existing antidepressant medication, which also slows down the metabolism of dextromethorphan in the body. Staying in the body longer means the levels of drugs are more stable and can potentially help patients more.
A phase three clinical trial confirmed that Auvelity can improve symptoms of depression and a few patients started experiencing the benefits after just one week. After six weeks, over 50% of the patients responded to the treatment and were feeling better and 40% were in remission which is a slightly higher remission rate than for other types of antidepressant medications. Crucially, this combination works significantly better than bupropion alone. After six weeks, only about 40% of patients responded to bupropion in isolation, and less than 20% achieved remission.
Auvelity doesn’t have the same safety restrictions as ketamine, making it a much safer alternative. Typically, it has similar side effects and warnings compared to existing antidepressant medications, including dizziness, nausea, dry mouth, decreased appetite, and anxiety.
There is a risk that Auvelity may increase suicidal thoughts and actions in young adults, adolescents, and children. For this reason, it’s essential to pay close attention to sudden changes in mood, behavior, thoughts, feelings, or suicidal thoughts when taking this drug.
Additionally, Auvelity should not be taken by patients with a history of seizures, or eating disorders, or those taking other antidepressants like monoamine oxidase inhibitors, benzodiazepines, barbiturates, or anti-seizure medicines.
Additional Considerations Regarding Traditional Antidepressant Medications
As described earlier, most antidepressants take weeks or months for patients to begin experiencing the benefits (if they experience benefits at all). However, there is some evidence that a few antidepressants work faster in some patients but there are no reliable ways to determine which patients will respond faster. These include, for example, some selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs).
SSRIs and SNRIs aim to increase the levels of serotonin and norepinephrine in the brain. They’re often the first option primary care doctors prescribe because they generally cause fewer side effects. Patients may experience better quality of sleep, higher levels of energy, and increased appetite within the first 1-2 weeks after starting treatment. Improvement in these physical symptoms can be an important early signal that the medication is working. In most cases, severe symptoms may still need up to 6-8 weeks to fully improve.
Some common examples of these drugs include:
- SSRIs
- Citalopram (Celesta)
- Fluoxetine (Prozac)
- Sertraline (Zoloft)
- SNRIs
- Venlafaxine (Effexor)
- Desvenlafaxine (Pristiq)
- Duloxetine (Cymbalta)
Similarly to SNRIs, tricyclic antidepressants also aim to increase the levels of serotonin and norepinephrine, but they tend to cause more side effects. They’re typically only prescribed for patients who have tried other antidepressants without improvement. One example of a fast-acting tricyclic antidepressant is called desipramine, and more than 50% of patients experience significant improvements during the first week of treatment.
However, it is important to note that response times to antidepressant medications vary greatly from patient to patient, and it is crucial to consult with a healthcare professional to determine the most appropriate treatment approach for an individual’s specific needs.
Conclusion
The treatment landscape for depression is evolving, with new options providing faster relief and potentially fewer side effects compared to traditional antidepressant medications. Traditional medications often involve a lengthy trial period and can come with unpleasant side effects, with only about one-third of patients having reliable reductions in depression symptoms.
Among the newer treatments, TMS stands out for its noninvasiveness and rapid effectiveness. Accelerated fMRI theta burst stimulation, in particular, offers a significant advancement by using fMRI and neuronavigation for precise coil placement, leading to high response and remission rates within a single week of treatment. This method, available at our clinic, is among the most effective fast-acting treatments for depression, with the added advantage of not impairing cognitive functions.
Other fast-acting treatments like ketamine, esketamine, and dextromethorphan-bupropion provide rapid symptom relief by targeting neurotransmitters such as glutamate. However, these treatments can come with side effects that patients should consider carefully, and the effects of these treatments are often not long-lasting.
Ultimately, the combination of fMRI TMS with cognitive behavioral therapy (CBT) offers a promising solution for many patients with treatment-resistant depression. This integrated approach not only provides rapid symptom relief but also promotes sustained improvement in brain function and overall mental health.
If you are interested in receiving accelerated fMRI TMS therapy at Cognitive FX, click here to learn more and see if you’re a good fit for treatment.
Cited Research