If you’re considering transcranial magnetic stimulation (TMS) therapy, one of the first and most important questions you’re likely to ask is: “Is TMS safe?”
The short answer is yes. TMS is a safe, FDA-approved procedure for treating major depressive disorder (MDD), as well as other conditions like obsessive-compulsive disorder (OCD), smoking cessation, and migraines.
Compared to antidepressant medications, TMS has fewer, milder, and shorter-lasting side effects, making it a compelling noninvasive treatment option for patients for whom medication has not worked or has led to unpleasant side effects. Furthermore, modern protocols of TMS are also faster-acting and significantly more effective than traditional antidepressant medications.
This article provides detailed information regarding what TMS is, its side effects, and its effectiveness. We also discuss how it compares to other depression treatment options, and offer perspective on interpreting negative TMS experiences that you may read about online.
We cover:
What Is TMS and How Safe Is It Really?
Transcranial magnetic stimulation (TMS) is a noninvasive, outpatient procedure that exposes the brain to a rapidly changing magnetic pulse. The brain region targeted in TMS is called the dorsolateral prefrontal cortex (DLPFC). This area is associated with our ability to feel positive feelings, including reward and motivation, and is the most likely to be affected by depression.
During a TMS session, an electromagnetic coil is positioned directly above the DLPFC on the patient’s head, and magnetic pulses are delivered at specific intervals to stimulate the nerve cells in that area. The aim is to increase activity in that region which, in turn, can alleviate symptoms in depressed patients.
The type of electromagnetic radiation emitted by a TMS coil is called non-ionizing radiation, which is harmless. In fact, we are constantly surrounded by electromagnetic radiation, including household appliances like microwave ovens, cellphones, Wi-Fi routers, computers, and washing machines. This type of radiation does not have enough energy to cause any kind of damage to our body, which makes TMS a very safe procedure. In addition, the magnetic pulses only reach a depth of about 1.5-3 cm beneath the scalp, which means its effect is limited to the target area and cannot cause any damage to other areas of the brain.
Who Is Not a Good Candidate for TMS?
The only patients for whom TMS may not be safe are patients with metallic implants (or other objects) in the brain close to where the coil needs to be placed. This includes, for example, cochlear implants, internal pulse generators, medication pumps, aneurysm clips or coils, stents, or even bullet fragments. (Patients with braces and dental fillings can undergo TMS safely.)
TMS represents a risk for these patients because magnetic pulses can induce large voltages in these devices, which can result in unintended brain stimulation. For example, TMS pulses can induce high voltages in the antenna in cochlear implants, which in turn can demagnetize its magnet, and damage the electronic chip. Magnetic pulses can also cause brain implants to overheat and cause irreversible damage to the brain. (In patients with no metal implants, the heating produced during a TMS session is negligible and poses no risk to the patient.)
TMS Safety for Children, Pregnant Women, and Elderly Patients
Most of the research has been done in adult patients, but TMS is generally accepted as safe for all patients, including children, pregnant women, and elderly patients.
- Children: A child’s brain is still developing and electromagnetic pulses may disrupt the nervous system and cause long-term impairments. Despite these concerns, multiple studies in children detected no serious or long-term side effects worse than what’s observed in adults. The major issue with young children is that their hearing may be affected more easily than adults. As they have smaller heads, the magnetic coil will be closer to their ear, and appropriate hearing protection devices are not always available for these young patients.
- Pregnant women: There is limited research regarding the use of TMS in pregnant women, but studies suggest that it is safe and effective. Magnetic fields dissipate rapidly with distance and are unlikely to reach the fetus. Encouragingly, babies born to mothers treated with TMS for depression during pregnancy didn’t experience perinatal complications and were within normal limits in both cognitive and motor development. Despite the promising results, the current recommendation is to take a conservative stance and analyze the risk/benefit ratio for each individual case.
- Elderly patients: Depression is common in elderly patients, but is challenging to treat, leading to a higher rate of patients with treatment-resistant depression compared to younger adults. As such, TMS is an attractive option for these patients. There were some early suggestions that elderly patients don’t respond as well and may need a high stimulation intensity to reach the targeted area, but current studies show that it’s an effective and safe treatment, with only mild and transient side effects.
Side Effects After TMS Are Mostly Mild and Short-Lived
In general, patients who receive TMS either don’t experience any side effects or experience only mild ones, such as temporary scalp discomfort or headaches. In addition, patients don’t need anesthesia or sedation and can go back to their normal daily activities immediately after each session.
Mild & Temporary Side Effects After TMS
Mild Scalp Discomfort
Scalp discomfort is the most commonly reported side effect after TMS, experienced by nearly 40% of patients. This happens because the magnetic pulses trigger some muscle contractions in the scalp, but usually dissipate shortly after the session. Patients can use painkillers to manage the symptoms if needed. In patients with intense pain, some of the treatment parameters can be adjusted to minimize discomfort during the initial sessions, such as placing the coil further away from the brain or changing the angle to ensure it doesn’t hit the trigeminal nerve or the scalp muscles that are causing the pain.
Headache and Localized Pain
Headaches are the second most common side effect of TMS, affecting about one-third of patients. Occasionally, the magnetic pulses may also stimulate some facial nerves and cause toothache, earache, twitching facial muscles, and neck and jaw pain, but these are less common. There is no evidence that TMS can cause migraines, even in patients with a history of this condition.
Typically, these symptoms are mild and rarely cause patients to stop their treatment. Clinical trials show that less than 2% of patients discontinue treatment due to pain. Patients who experience any headaches after their treatment can use over-the-counter painkillers such as ibuprofen or paracetamol. In most cases, patients get used to the procedure and the pain dissipates after a few sessions.
Hearing Changes
A less common side effect of TMS is an increased sensitivity to sound, which occurs when the magnetic pulses stimulate areas involved in auditory processing. Some patients become more aware of ambient noises or hear sounds more intensely than before treatment. In most cases, this is only temporary, and normal hearing returns after treatment. In rare cases, TMS can trigger tinnitus and hearing loss, especially if patients undergo TMS sessions without earplugs. To avoid hearing problems, make sure you always wear ear protection during treatment. Studies confirm this is the best way to prevent changes in hearing after TMS. If you experience any significant changes for a prolonged period, seek medical help to assess for hearing loss and tinnitus.
Possible (but Rare) Serious Side Effects After TMS
Seizures
The most serious side effect during TMS treatment is seizures, but the risk is minimal for most patients. Most of the cases of seizures occurred in the early days of TMS. Since then, many safety features have been added to the procedure, significantly reducing the risk for patients. Current estimates suggest that less than 3 patients experience a seizure per 100,000 sessions. Patients are more likely to experience seizures during the initial sessions, but they can occur later in the course of treatment, particularly if there are clinical or medication changes.
Seizures are more likely in patients with a history of seizures or suffering from neuropsychiatric diseases, including epilepsy, multiple sclerosis, traumatic brain injury, and Alzheimer’s disease. In addition, patients who routinely experience poor sleep, drink excessively, take prescription drugs that lower the seizure threshold, or are suffering from high levels of stress also have a higher risk of seizures. The good news is that seizures tend to be isolated events and don’t cause serious long-term consequences. There is no evidence that patients may experience multiple seizures during or after TMS sessions.
Syncope
It’s not unusual for patients to feel lightheaded and faint during a TMS session. This is not caused by the TMS procedure itself. Rather, it is a result of anxiety and stress. Syncope is not serious in itself, but occasionally, it may look like patients are having a seizure and it can be very distressing.
Acute Psychological Changes
Some patients have reported unexpected emotional responses, including exaggerated mood swings, anxiety, agitation, insomnia, manic episodes, and suicidal ideation. Studies show these mental health changes affect less than 1% of patients and occur especially in patients with bipolar disorder and taking antidepressant medication for major depressive disorder. These responses seem to dissipate shortly after the treatment but patients should still seek specialist medical care if they encounter this.
How Effective Is TMS Therapy for Treating Depression?
The standard form of TMS (known as repetitive TMS or rTMS) has demonstrated effectiveness in improving depression symptoms in approximately 50% of patients, with over 30% achieving remission (i.e., a significant reduction or disappearance of depression symptoms). When combined with psychotherapy, success rates are even more impressive with remission and response rates of ~55% and ~66%, respectively.
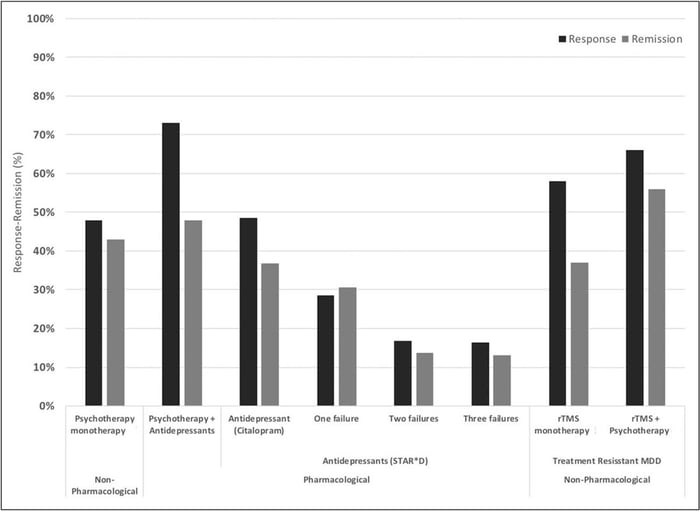
Response and remission rates of various monotherapeutic and combinatory antidepressant treatments based on the largest studies and datasets available. [Source]
Compared to antidepressant medications, these are significant improvements. However, a newer form of TMS —accelerated, fMRI-guided theta burst stimulation — is producing even better results.
Accelerated fMRI-Guided Theta Burst Stimulation Is Producing the Best Treatment Outcomes to Date
In 2018, the US Food and Drug Administration approved a novel form of accelerated TMS known as Intermittent Theta-Burst Stimulation (iTBS). Theta waves, which the hippocampus uses to connect with other brain circuits, play a role in memory formation, meditation, and certain phases of sleep.
With iTBS, many patients notice improvements soon after beginning treatment. Additionally, there is a reduction in suicidal ideation, indicating that iTBS may be a rapid treatment option for patients at high risk of suicide.
The SAINT™ protocol, a revolutionary TMS treatment developed at Stanford University, combines theta-burst stimulation with functional MRI and neuronavigation to ensure precise coil placement over the specific area of the brain targeted by TMS. This represents a significant advancement.
In traditional rTMS and iTBS methods, the coil’s placement is determined by manually measuring distances between the patient’s nose, ears, and the top of the head. However, due to variations in head size, shape, and individual brain organization, this method can be imprecise and result in less consistent outcomes.
With the use of fMRI and neuronavigation, healthcare practitioners can accurately position the magnetic coil over the dorsolateral prefrontal cortex (DLPFC) for each patient. This approach is now considered the “gold standard” for treating treatment-resistant depression.

A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
In a double-blind randomized controlled clinical trial, about 85.7% of patients responded to SAINT treatment, meeting predefined criteria for reduced depressive symptoms, while around 78.6% achieved remission.
All participants had treatment-resistant depression and had failed at least two other depression treatments. Encouragingly, one month after treatment, 60% of patients remained in remission.
The rapid achievement of high response and remission rates with fMRI-guided theta burst stimulation, combined with minimal side effects, makes it one of the most effective treatments available today. However, there is limited data on the long-term durability of these outcomes. While some patients may experience lasting remission or symptom reduction, others might require follow-up treatment to maintain effectiveness.
As such, combining TMS with cognitive behavioral therapy (CBT), which has been shown to produce strong long-term outcomes, is likely to offer the best results for patients.
Receiving Accelerated fMRI-Guided TMS for Depression at Cognitive FX
Our clinic in Provo, Utah, provides an alternative to SAINT™ TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT™ (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
This accelerated protocol of iTBS is:
- Safe: Widely tolerated and associated with mild, short-lasting side effects.
- Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
- Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work commitments (compared to 4 to 6 weeks of standard TMS and accelerated TMS protocols).
To improve patient outcomes, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.
How TMS Compares to Other Depression Treatment Alternatives
We’ve written several in-depth articles covering how TMS compares to other depression treatment alternatives such as electroconvulsive therapy (ECT) and ketamine. Each comparison is made in terms of effectiveness, side effects, cost, and accessibility.
Find those articles here:
Perspective on Interpreting Negative Online Comments About TMS Experiences
You may encounter negative comments about the procedure when researching TMS safety online. You might find blog posts claiming that TMS damages the brain, websites where patients share negative experiences, or Reddit posts with patients asking genuine questions about managing their symptoms or whether TMS is suitable for them.
While finding others with similar experiences can be comforting, please remember that those participating in these discussions are not medical professionals. For questions about TMS and its side effects, consult your healthcare provider.
We acknowledge that some patients have had negative experiences with TMS, but these cases are rare. Most of the thousands who have undergone TMS have seen significant improvements with mild side effects and have returned to their normal lives without posting online.
With that said, here we’ll offer some perspective on the following online comments regarding TMS experiences:
Increase anxiety after the first session?
Anyone experienced increased anxiety after the first session? The doctors mentioned something (after two failed meds Abilify and Rexulti) about activation. Wonder if that has anything to do with it?
It’s not unusual for patients to feel worse after the initial sessions before they start to feel better. This is known as a TMS dip and it’s usually short-lived.
As we’ve seen, TMS uses electromagnetic pulses to stimulate specific areas of the brain with healthier levels of electrical activity. This triggers nerve cells to release neurotransmitters and establish new neural pathways, allowing the brain to adopt new communication patterns that positively affect thinking and behavior. However, this process takes time, and sometimes the brain remains "stuck" in old patterns, causing temporary worsening of symptoms.
It’s generally advised to stick with the treatment, even if you don’t feel better immediately. However, it’s important to discuss your experience with your practitioner. In some cases, such as instances of extreme increases in anxiety, it may make sense to postpone remaining treatment sessions or end treatment early.
Gwen’s story
Soon after finishing treatment, I began to experience pain in my upper back teeth, closest to where the machine sat on my head. I could also feel all of the other teeth in my mouth. It felt like I had pebbles in my mouth. […] Some of my other side effects included intense tinnitus, electrical jolts down my calves, rapid heart rate, and restless legs syndrome throughout my entire body. […] I was prescribed more anti-anxiety and depression meds. I was always sensitive to meds but even more so after TMS. I couldn’t tolerate the side effects, the meds made me feel like I was jumping out of my skin. My body felt electrified. The doctors had no idea how to help me.
Angela’s story
Initially during the first few weeks of treatment I started to feel better. […] Treatment ended with about 6 weeks of sessions due to another issue of getting insurance approval. Since this time my symptoms have continued to progress to a terrible state. I have no energy whatsoever. Every daily task is exhausting and overwhelming to me. I’m having continued suicide ideation that’s overwhelming at times because I’m so overwhelmed and miserable with my symptoms. My anxiety puts me in manic states of uncontrollable spending and racing thoughts.
As it goes with any type of depression treatment, some portion of patients will not respond or achieve remission with TMS. Sadly, Gwen and Angela seem to have experienced multiple side effects with limited long-term benefits.
However, it’s important to consider that these experiences are rare even for the original and most widely available protocol of TMS, rTMS. With the accelerated fMRI-guided theta burst protocol that we offer at our clinic, the most serious side effects associated with this treatment are mild headaches and fatigue after the treatment. It is also proven to be a good option for patients at high risk of suicide.
Finally, Gwen and Angela may not have received the best support during their treatment. At Cognitive FX, we combine our TMS sessions with cognitive behavioral therapy (CBT) to ensure that our patients receive the help they need.
When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. We believe CBT is likely to produce similar — if not better — improvements for our patients.
Day 28. I’m a mess.
Seriously, I have lost all hope. Zero improvement of major depression symptoms [after TMS]. Cried all morning through treatment and all day at work. Filled out the Spravato paperwork this morning to get preauth through insurance for treatment. I truly cannot believe this didn’t help. Obviously I’m finishing out through session 36, but what a failure.
Day 28 is not the end of the treatment and some patients only experience significant improvements after the treatment is completed. Our advice would be to complete the TMS sessions and wait for a while, instead of jumping so soon to another treatment.
Key Takeaways
- TMS is FDA-approved and safe: TMS is a non-invasive, FDA-approved treatment for major depressive disorder (MDD) and other conditions like OCD, smoking cessation, and migraines. It uses non-ionizing electromagnetic radiation, which is harmless and does not cause damage to the body.
- Fewer side effects compared to medications: TMS has fewer and milder side effects than antidepressant medications. Common side effects include temporary scalp discomfort, headaches, and increased sound sensitivity, all of which are generally mild and short-lived.
- TMS is equally or more effective than medications: The standard rTMS protocol achieves significant symptom improvement in about 50% of patients and remission in about 30%. Newer fMRI-guided theta burst protocols show significantly higher success rates. 85.7% of patients responded to this one-week treatment in a double-blind randomized controlled trial, with 60% of patients still in remission one month later. The long-term durability of these protocols is still being studied.
Cited Research