Although transcranial magnetic stimulation (TMS) has been used for over 20 years and FDA-approved for treating major depressive disorder since 2008, it remains relatively less well-known among the general public. Despite this, it is regarded as one of the safest and most effective treatment options available today.
If you’re considering or have heard about TMS therapy for treatment-resistant depression (TRD), you likely have questions about what it involves, what the risks and side effects are, how effective it is, and more.
In this article, we provide a comprehensive overview of TMS to inform patients and their loved ones about this evidence-based treatment and help them better understand if it would be a good fit for them.
We cover:
What Is TMS Therapy for Depression?
TMS is a non-invasive outpatient procedure that uses magnetic fields to stimulate a part of the brain that’s consistently impacted by major depression. The brain region it targets is called the dorsolateral prefrontal cortex (DLPFC), which is associated with a patient’s ability to feel positive feelings such as reward and motivation.
During a TMS treatment session, an electromagnetic coil is positioned over the patient's head, directly above the DLPFC, and magnetic pulses are delivered at specific intervals to stimulate the nerve cells in that area. The result is an increase in activity in that region which can help alleviate symptoms and improve mental health in depressed patients.
Note: Research is ongoing into other potential uses for TMS, including conditions such as epilepsy, addictions, Alzheimer's disease, and bipolar disorder.
How Does TMS Therapy Work?
The original and most widely used form of TMS is known as repetitive transcranial magnetic stimulation or rTMS. Standard treatments involve daily sessions, five days a week, with 20-30 sessions delivered over 4-6 weeks. Some patients see improvements after 2-4 weeks, while others only see the benefits after treatment is completed.
The primary drawback of repetitive TMS is the length of time required for the treatment to be completed. Many patients find it challenging to adhere to a 6-week treatment plan due to family and work obligations. This challenge prompted researchers and clinicians to develop accelerated TMS, where patients attend multiple sessions per day, reducing the treatment course to just one week. This shorter time-frame makes it more feasible for patients to complete the treatment.
Studies show accelerated TMS is as safe as daily rTMS and potentially more effective. Patients receiving multiple sessions each day tend to experience improvements in symptoms faster than patients receiving daily sessions, and the effects are still visible months after their treatment.
What Are the Potential Risks and Side Effects of TMS?
TMS is associated with relatively few side effects, risks, and complications. The most likely side effects are mild and usually don’t last more than a few minutes after each session. Often, patients get used to the procedure after a few sessions, and side effects dissipate.
Common side effects may include:
- Headaches
- Pain, usually in your scalp or neck
- Dizziness or lightheadedness
- Facial or scalp tingling
- Temporary tinnitus (ringing in your ears)
- Unusually high sensitivity to sound
The most serious potential side effect of TMS is a seizure, but these are extremely rare. The risk of having a seizure from a TMS session is less than 0.01% per session or less than 1 in 10,000 sessions.
How Effective is TMS Therapy for the Treatment of Depression?
The standard form of TMS (rTMS) has demonstrated effectiveness in improving depression symptoms in approximately 50% of patients, with over 30% achieving remission (i.e., a significant reduction or disappearance of depression symptoms). When combined with psychotherapy, success rates are even more impressive with remission and response rates of ~55% and ~66% respectively.
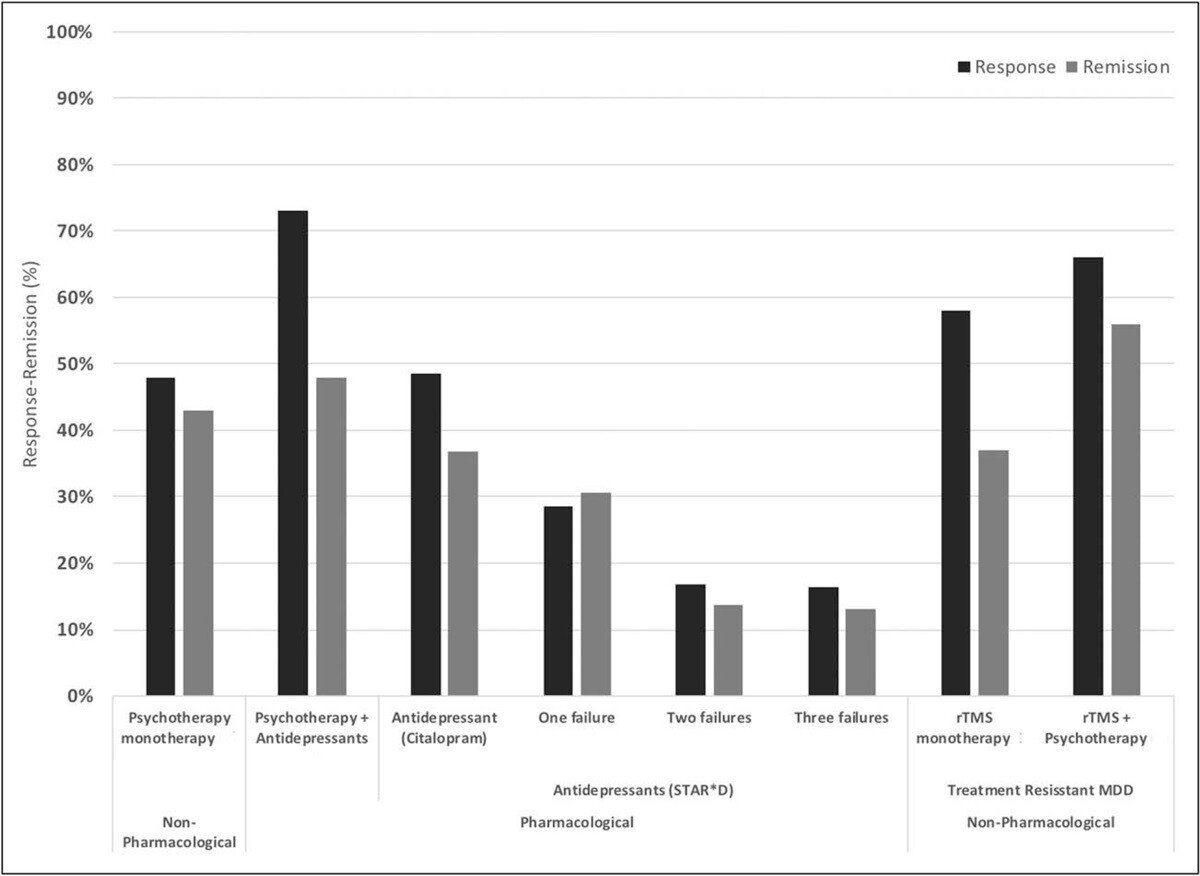
Response and remission rates of various monotherapeutic and combinatory antidepressant treatments based on the largest studies and datasets available. [Source]
Already, these are significant improvements in effectiveness compared to antidepressant medications. However, a new form of TMS, the SAINT™ protocol (Stanford Accelerated Intelligent Neuromodulation Therapy), is yielding even better outcomes.
SAINT™ — a Stanford-Developed TMS Protocol — is Producing the Best Treatment Outcomes to Date
In 2018, the US Food and Drug Administration approved a novel form of accelerated TMS known as Intermittent Theta-Burst Stimulation (iTBS). Theta waves, which the hippocampus uses to connect with other brain circuits, play a role in memory formation, meditation, and certain phases of sleep.
iTBS employs a different magnetic pulse pattern (delivered in triplets) and administers treatment in three minutes, compared to the 37 minutes required for rTMS. In addition, it applies pulses at 80% of the brain’s motor threshold (compared to high-frequency pulses of 110-120% with rTMS), making it even safer for patients.
Many patients notice improvements soon after beginning treatment. Additionally, there is a reduction in suicidal ideation, indicating that iTBS may be a rapid treatment option for patients at high risk of suicide.

The SAINT protocol combines theta-burst stimulation with functional MRI and neuronavigation to ensure precise coil placement over the specific area of the brain targeted by TMS. This represents a significant advancement.
In traditional rTMS and iTBS methods, the coil’s placement is determined by manually measuring distances between the patient’s nose, ears, and the top of the head. However, due to variations in head size, shape, and individual brain organization, this method can be imprecise and result in less consistent outcomes.
With the use of fMRI and neuronavigation, healthcare practitioners can accurately position the magnetic coil over the dorsolateral prefrontal cortex (DLPFC) for each patient. This approach is now considered the “gold standard” for treating treatment-resistant depression.
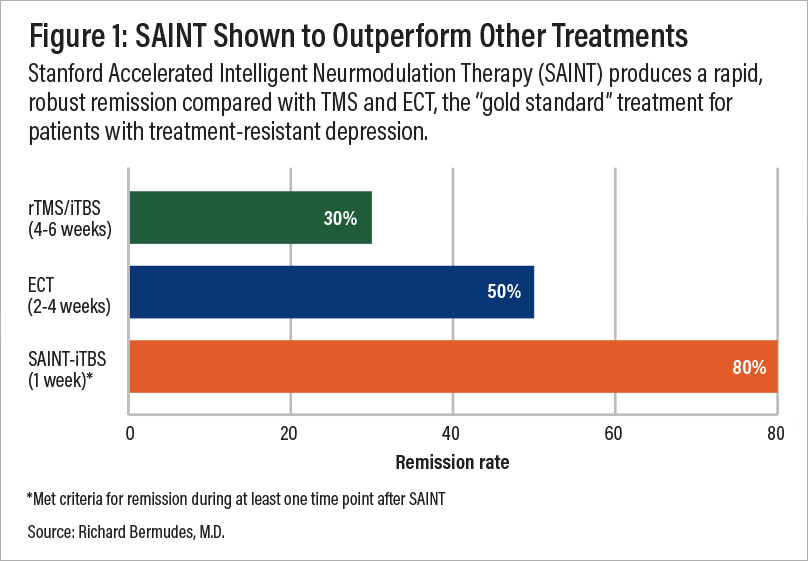
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
In a double-blind randomized controlled clinical trial, about 85.7% of patients responded to the SAINT-iTBS treatment, meeting predefined criteria for reduced depressive symptoms, while around 78.6% achieved remission. All participants had treatment-resistant depression and had failed at least two other depression treatments. Encouragingly, one month after treatment, 60% of patients remained in remission.
The rapid achievement of high response and remission rates with SAINT™, combined with minimal side effects, makes it one of the most effective fast-acting treatments available today. However, there is limited data on the long-term durability of these outcomes. While some patients may experience lasting remission or symptom reduction, others might require periodic follow-up treatments to maintain effectiveness.
As such, combining TMS with cognitive behavioral therapy (CBT), which has been shown to produce strong long-term outcomes, is likely to offer the best results for patients.
Who Is a Good Fit for TMS Therapy?
TMS is suitable for most patients, but we recommend this treatment especially for patients who:
- Have tried multiple rounds of different antidepressant medications and psychotherapy with little to no relief from their symptoms
- Wish to avoid the side effects of antidepressant medications
- Wish to avoid more severe treatments, such as electroconvulsive therapy (ECT), which is associated with more serious side effects
- Are between the ages of 18 and 65 years old
Who Should Avoid TMS Therapy?
There are not many restrictions associated with TMS, but this intervention may not be suitable for some patients, including:
- Those with metal objects in the brain, such as deep brain stimulators, aneurysm clips or coils, shrapnel or bullet pieces, facial tattoos with metallic ink, metal plates, cochlear implants, and permanent piercings (braces and dental fillings are acceptable)
- Those dealing with suicidal thoughts or have attempted suicide recently
- Those with a seizure disorder or a history of seizures
- Those taking certain antidepressant or stimulant medication
In some cases, it may be possible for patients to still receive TMS even if they fit into one of the above categories. If this applies to you, discuss your suitability with your doctor.
What Are TMS Costs and Insurance Coverage Like?
The cost of TMS therapy can vary greatly based on geographic location, provider expertise, the type of TMS used, and insurance coverage. In general, the cost of a TMS treatment course ranges from $6,000 to $15,000 without insurance. For more advanced protocols, costs can increase significantly. For example, clinics offering SAINT™ treatment charge $30,000+.
With insurance, out-of-pocket costs can be significantly reduced. However, depending on your provider and specific policy, you may need to have tried two to four antidepressant medications and/or therapy with a psychiatry specialist before you can qualify for TMS coverage.
The alternative is to seek out clinics that offer an accelerated TMS protocol, which can be completed in a single week.
Where Can You Receive TMS Therapy?
Particularly in urban areas, it can be relatively easy to find clinics that offer the traditional protocol of rTMS, which requires patients to participate in daily sessions, five days a week for 4-6 weeks. However, as we discussed above, this can be a challenging treatment to pull off for many people due to work and other life commitments.
The alternative is to seek out clinics that offer accelerated TMS, which can be done in a single week.
Receiving Accelerated fMRI-TMS at Cognitive FX: A SAINT™ Alternative for One-Third of the Price
Our clinic, based in Provo, Utah, provides an alternative to SAINT TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT™ (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
This protocol of TMS is:
- Safe: Widely tolerated and associated with mild, short-lasting side effects.
- Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
- Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work commitments (compared to 4 to 6 weeks of standard TMS and accelerated TMS protocols).
To improve outcomes for our patients, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.
Cited Research