Is TMS Safe? Answers for Patients Considering TMS Therapy
If you’re considering transcranial magnetic stimulation (TMS) therapy, one of the first and most important questions you’re likely to ask is: “Is TMS safe?”


Transcranial magnetic stimulation (TMS) and neurofeedback are gaining popularity as non-invasive, medication-free options for treating depression—especially for people who haven’t found relief from antidepressant medications. While both aim to improve brain function as a means to reduce depression symptoms, they take very different approaches.
In this article, we explain how each treatment option works, compare their effectiveness, side effects, and costs, and discuss a promising alternative that builds on their strengths while addressing their limitations.
We cover:
TMS is a non-invasive therapy that uses magnetic fields to stimulate a specific area of the brain called the dorsolateral prefrontal cortex (DLPFC)—a key brain region involved in mood, emotion regulation, and decision-making.
During treatment, a technician places an electromagnetic coil over your scalp, targeting the DLPFC. The coil delivers a series of magnetic pulses that stimulate neuronal activity in this region. This stimulation helps "reboot" dysfunctional circuits in the brain that are associated with depression and other mental health disorders.
TMS has been used to treat depression for nearly 20 years. The original and most common TMS protocol, called repetitive TMS (rTMS), is administered for about 30–40 minutes per session, five days a week, for four to six weeks.
To shorten treatment time, researchers developed a newer method of TMS called intermittent theta burst stimulation (iTBS). Instead of simple repetition, iTBS mimics natural brain rhythms using quick bursts of pulses.
Each session lasts just 3–10 minutes, allowing accelerated TMS protocols with multiple daily sessions to complete treatment in as little as one week — without sacrificing effectiveness.
Neurofeedback is a form of brain training that helps you self-regulate brain activity in real time. It most commonly uses EEG sensors (sometimes called EEG biofeedback), where electrodes track your brainwave patterns and translate them into audio or visual signals. For example, a video might change when your brain reaches a desired state, reinforcing that activity.
Neurofeedback training relies on operant conditioning: When you receive positive feedback for healthy brain patterns, you're more likely to repeat them.
A typical neurofeedback session lasts 30 to 60 minutes, with the number of sessions varying by individual. Neurofeedback is also used as an intervention for mental health conditions such as anxiety, attention-deficit/hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD).
|
Factor |
TMS |
Neurofeedback |
Mechanism |
Stimulates DLPFC with magnetic pulses |
Real-time brain activity feedback via EEG or fMRI |
Session Duration |
3–40 minutes (depending on protocol) |
30–60 minutes |
Total Treatment Time |
Daily TMS sessions for 4–6 weeks; shorter with accelerated protocols |
1–2 sessions/week, varies per patient |
Patient Involvement |
Passive |
Active—requires participation and learning |
Efficacy |
Strong clinical evidence for treatment-resistant depression (~50% response, ~30% remission) |
Emerging evidence (40–50% remission in small studies) |
Side Effects |
Mild discomfort, headache; rare risk of seizure |
Mild fatigue, headache; rare adverse responses if protocols are misapplied |
Contraindications |
Not suitable for patients with certain implants or seizure history |
Not recommended for patients with neurological injuries or frequent migraines |
FDA Status |
FDA-approved for major depressive disorder |
FDA-cleared as diagnostic tool, not specifically for treating depression |
Cost |
$6,000–$15,000 (may be covered by insurance); $30,000+ for the most advanced accelerated protocols (not covered by insurance) |
$1,500–$8,000 (insurance coverage varies) |
Availability |
Widely available for rTMS; iTBS less common |
Available in many clinics—certification varies |
TMS has a strong track record of success for people who haven’t responded to medications. Robust scientific research shows:
While some patients see major improvements, neurofeedback research is still in its early stages. Key points to know:
Lengthy treatment: rTMS requires daily sessions over 4–6 weeks, making it difficult to complete alongside work and life commitments.
Lack of precision: Coil placement relies on manual measurement, leading to inconsistencies affecting treatment effectiveness.
Moderate success rates: While rTMS has higher response and remission rates than antidepressant medications, only about one-third of patients achieve full remission.
Limited scientific evidence supports neurofeedback as an effective depression treatment.
No standard protocol exists, and it's impossible to predict who will respond well.
Lengthy treatment: Neurofeedback requires 20 to 40 sessions over 10+ weeks to complete a treatment course.
In 2022, Stanford University researchers developed a TMS protocol combining theta-burst stimulation with functional MRI and neuronavigation technology to improve precision and speed at which patients achieve symptom relief.
This FDA-approved protocol, known as SAINT™ TMS (Stanford Accelerated Intelligent Neuromodulation Therapy), is now considered the gold standard for treatment-resistant depression, shortening the TMS treatment schedule to a single week and significantly increasing response and remission rates compared to conventional rTMS.
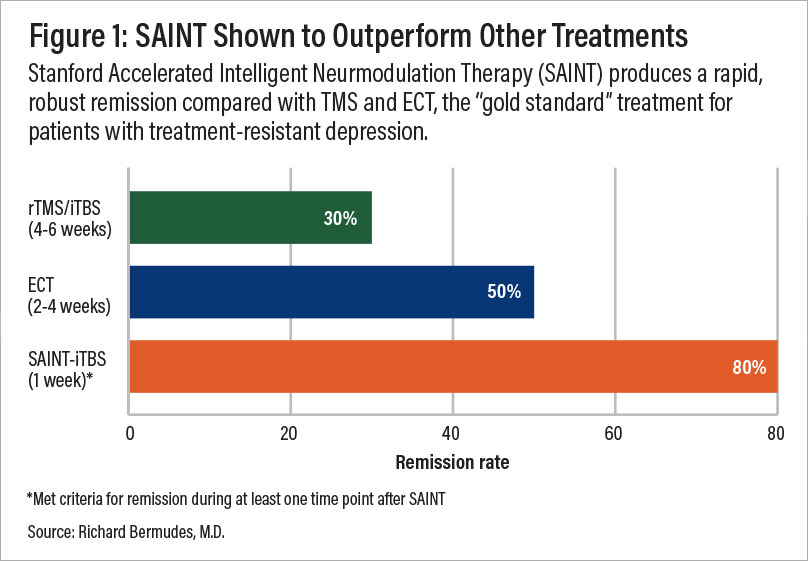
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
In a double-blind randomized clinical trial, about 86% of patients responded to SAINT treatment, with around 79% reaching remission. All participants had treatment-resistant depression and had failed at least two other depression treatments.
One month after treatment, 60% were still in remission.
In addition, this protocol quickly reduces depressive symptoms and suicidal ideation within 5 days, without serious adverse side effects.
Many patients face practical barriers to receiving SAINT™ treatment, such as insurance coverage limitations, cost, or geographical constraints. If this applies to you, talk with your provider to explore alternative options.
While SAINT offers many advantages over traditional TMS, several factors limit accessibility:
Lack of insurance coverage: Most insurance plans do not yet cover SAINT, as it is a newer protocol than standard TMS. However, coverage policies vary, so it’s important to check with your insurance provider to confirm your options. As of July 1, 2025, the Centers for Medicare and Medicaid Services (CMS) began reimbursing hospitals $19,703 for the full SAINT protocol, which may help expand coverage over time.
High treatment costs: Currently, SAINT TMS treatments range from $30,000 to $36,000, making cost a significant barrier for many. However, there are alternatives available at a significantly lower price (discussed below).
Limited availability: Fewer than 10 clinics in the U.S. currently offer SAINT; most still use rTMS. However, more clinics are adopting accelerated TMS and intermittent theta burst stimulation (iTBS) protocols, signaling a gradual shift toward newer treatment methods.
If you’re considering SAINT treatment, discussing financial and logistical concerns with a qualified healthcare professional can help you explore potential solutions like financing options or alternative treatment locations.
Our clinic, based in Provo, Utah, provides an alternative to SAINT TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
| Accelerated fMRI - TMS | Magnus SAINT™ TMS | |
|---|---|---|
| FDA-Approved iTBS | ✔ | ✔ |
| FDA-Approved Neuronavigators | ✔ | ✔ |
| FDA-Approved Figure 8 Coils | ✔ | ✔ |
| Number of Treatment Days | 5 | 5 |
| Treatments per Day | 10 | 10 |
| Total Treatments | 50 | 50 |
| Number of TMS Pulses | Approx. 90,000 | 90,000 |
| Resting motor threshold pulse intensity | 90–120% | 90–120% |
| FDA-Approved Personalized DLPFC Targeting | ✘ | ✔ |
| Personalized DLPFC Targeting Assists Doctor in Target Location | ✔ | ✘ |
| Personalized E Field Coil orientation | ✔ | ✘ |
| Cost | $9,000 to $12,000 | $30,000+ |
Our TMS protocol is:
Safe: Widely tolerated and associated with mild, short-lasting side effects.
Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work.
Effective: Precision coil placement combined with theta burst stimulation produces the best TMS treatment results to date.
To improve outcomes for our patients, we include cognitive behavioral therapy (CBT) as part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Furthermore, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.
Both TMS and neurofeedback offer promising non-drug options for treating depression.
TMS is best for patients with treatment-resistant depression or those needing rapid results. fMRI-guided accelerated TMS offers significant advantages in speed and effectiveness.
Neurofeedback may appeal to those seeking a hands-on approach to brain training and resilience-building.
If you’re looking for fast, lasting relief with precision targeting, consider accelerated fMRI-guided TMS. It combines the strengths of both proven technology and personalization—offering the best odds of alleviating symptoms and feeling like yourself again.
Always consult with a qualified provider before starting treatment. If you’d like to learn more about our Provo-based program, take our quiz to see if you’re a good fit for treatment.

Dr. Mark D. Allen holds a Ph.D. in Cognitive Science from Johns Hopkins University and received post-doctoral training in Cognitive Neuroscience and Functional Neuroimaging at the University of Washington. As a co-founder of Cognitive Fx, he played a pivotal role in establishing the unique and exceptional treatment approach. Dr. Allen is renowned for his pioneering work in adapting fMRI for clinical use. His contributions encompass neuroimaging biomarkers development for post-concussion diagnosis and innovative research into the pathophysiology of chronic post-concussion symptoms. He's conducted over 10,000 individualized fMRI patient assessments and crafted a high-intensity interval training program for neuronal and cerebrovascular recovery. Dr. Allen has also co-engineered a machine learning-based neuroanatomical discovery tool and advanced fMRI analysis techniques, ensuring more reliable analysis for concussion patients.

If you’re considering transcranial magnetic stimulation (TMS) therapy, one of the first and most important questions you’re likely to ask is: “Is TMS safe?”

Intermittent theta burst stimulation (iTBS) has been growing in popularity since receiving FDA approval for treating major depression in 2018. This new brain stimulation method uses a series of...

Traditional antidepressant medications involve a trial period of weeks or months before it can be determined whether or not they are working. If a medication doesn’t work — and it’s been shown that ...

If you or a loved one have had failed attempts with traditional antidepressant drugs, you may be considering alternative treatment options such as electroconvulsive therapy (ECT) and transcranial...

A major challenge for patients with depression is that traditional antidepressant medications often take weeks or months to show results — if they work at all. This delay can be especially...

While antidepressant medications remain the most common first-line treatment for depression, research shows they only work for about one-third of patients and often come with unpleasant side effects...