Accelerated TMS Therapy: Complete Treatment Overview
If you’re considering accelerated Transcranial Magnetic Stimulation (TMS) therapy, it’s important to understand how it works, what to expect, and whether it’s the right fit for you.

If you’re considering transcranial magnetic stimulation (TMS) therapy, it’s essential to understand the potential pros and cons of the treatment. Fortunately, for those suffering from major depressive disorder (MDD), the pros far outweigh the cons for many patients.
Compared to antidepressant medications, TMS has fewer, milder, and shorter-lasting side effects, making it an appealing noninvasive treatment option for patients who haven’t responded to medication or have experienced unpleasant side effects. Modern TMS protocols are also faster-acting and more effective than traditional antidepressant medications.
Below, we outline the main pros and cons of TMS treatment to help you determine if it’s a viable option for you. Specifically, we cover:
Transcranial Magnetic Stimulation (TMS) targets a specific part of the brain called the dorsolateral prefrontal cortex (DLPFC) — a region crucial for mental health, including mood, emotion regulation, and decision-making.
During a TMS session, an electromagnetic coil is positioned over the patient’s head directly above the DLPFC, delivering magnetic pulses at specific intervals to stimulate the nerve cells in that area. This increased activity in the DLPFC can help alleviate depression symptoms for many patients.
TMS has been used to treat depression for nearly 20 years. The original method of TMS, called repetitive transcranial magnetic stimulation (rTMS), is typically administered once a day for about 30 minutes, five days a week for four to six weeks. However, this treatment can be challenging for patients with full-time jobs or family commitments.
To overcome this issue, researchers developed an accelerated TMS protocol where patients receive multiple daily sessions, shortening the course of treatment to just one week. This condensed timeframe makes it easier for patients to complete the treatment, and the new protocol is as effective as rTMS.
In 2018, researchers introduced a novel form of accelerated TMS known as Intermittent Theta-Burst Stimulation (iTBS). iTBS uses a different magnetic pulse pattern delivered in triplets and administers treatment in just three minutes, compared to the 37 minutes required for rTMS. This technique was combined with functional MRI and neuronavigation for precise coil placement over the targeted area of the brain. This new Stanford-developed protocol, SAINT™ treatment, involves five days of 10 intermittent theta-burst stimulation sessions per day.
One of the main benefits of TMS is its effectiveness, especially compared to traditional antidepressant medications, which help only about one-third of patients who take them.
The original form of TMS, rTMS, can improve symptoms in about 50% of patients with depression, with over 30% achieving remission. TMS is particularly effective in patients with mild depressive episodes and non-psychotic depression. While patients who have not responded to multiple antidepressant medications are more challenging to treat, around 30% still respond to treatment, and 19% achieve remission. Overall, when combined with psychotherapy, success rates are even higher, with remission and response rates of ~55% and ~66%, respectively.
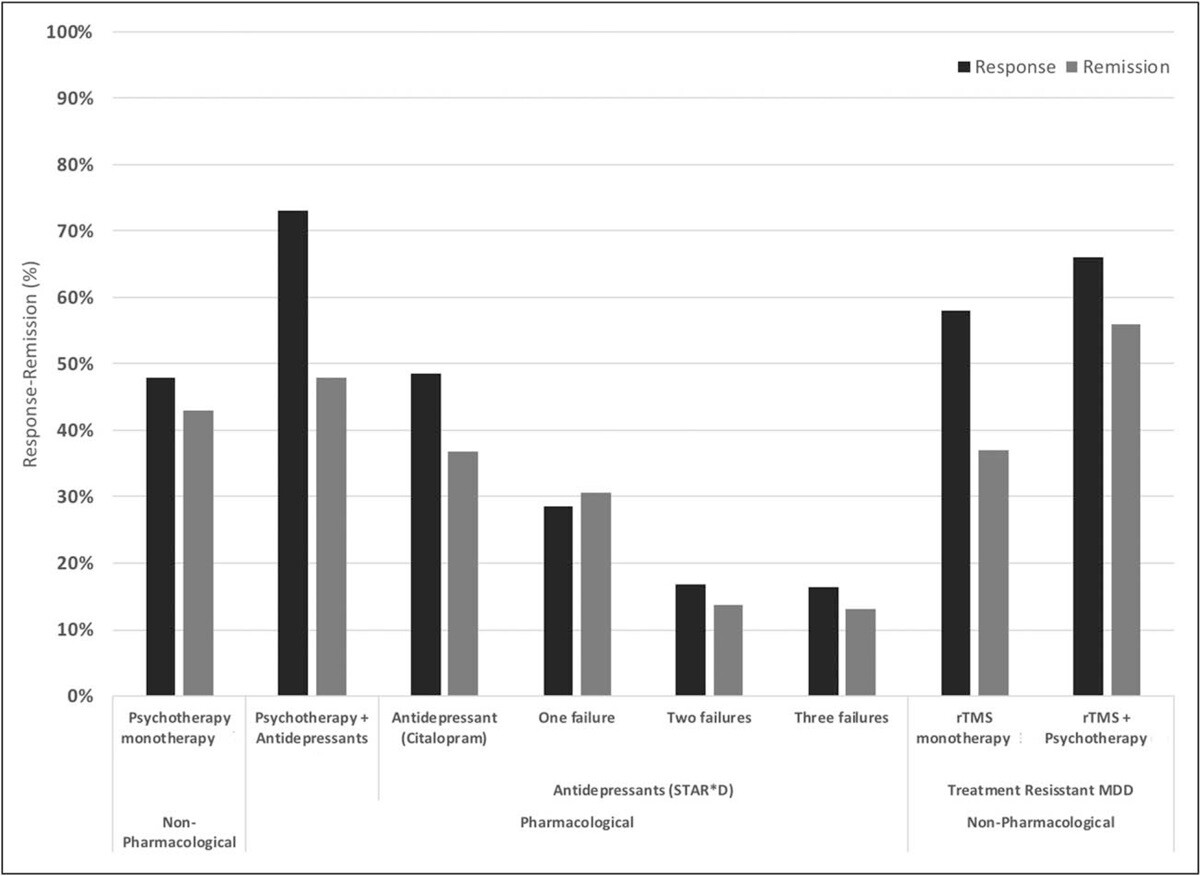
Response and remission rates of various monotherapeutic and combinatory antidepressant treatments based on the largest studies and datasets available. [Source]
These results are already promising, but new accelerated fMRI-guided theta burst protocols are proving to be even better. With this method, 90% of patients who previously didn’t respond to conventional TMS achieved remission in just three to five days.
In a randomized controlled trial with patients never treated with TMS, about 85% responded to the treatment, and around 78% achieved remission within five days of treatment. About four weeks later, remission remained around 60%.
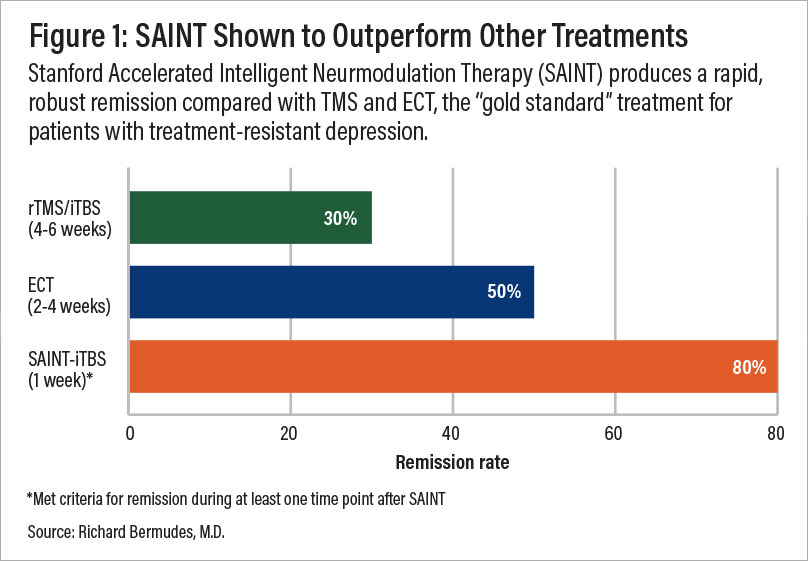
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
TMS is a safe and well-tolerated procedure, and most patients experience only mild and short-lived side effects. The most common side effects include headaches, dizziness, and some discomfort at both the stimulation site and in the facial muscles during treatment.
Other less common (but still mild) side effects include gastrointestinal discomfort, muscle twitching, dizziness, insomnia, tinnitus, or fainting. The most serious potential side effect of TMS is a seizure, but these are extremely rare. The risk of having a seizure from a TMS session is less than 0.01% per session or less than 1 in 10,000 sessions.
Many patients notice improvements soon after beginning their TMS sessions. Additionally, iTBS can reduce suicidal ideation, indicating that it may be a rapid treatment option for high-risk patients. This is in stark contrast to antidepressant medications, which can take 3–6 weeks or more to begin helping patients.
Like any other treatment for mood disorders, some patients may relapse after TMS treatment. However, most TMS patients feel better for many months after treatment ends and about two-thirds of those who achieve remission with rTMS treatment remain symptom-free after 12 months. This is especially true when patients receive maintenance TMS sessions as needed to prevent the return of symptoms.
Accelerated fMRI-guided theta burst stimulation, as per the SAINT™ protocol, has not been thoroughly evaluated to determine how long its effects last, on average. Still, preliminary results suggest that around 50% of patients remain in remission 6 months after treatment. We believe that maintenance sessions and/or cognitive behavioral therapy (CBT) can further improve these outcomes.
In contrast to other alternative depression treatments, such as electroconvulsive therapy (ECT), TMS is a non-invasive outpatient procedure. Patients do not need to undergo anesthesia, sedation, or receive medication during their treatment. The TMS device operates completely outside the body and affects the brain system with magnetic pulses generated in a magnetic coil simply placed over the patient’s head.
Another advantage of TMS is that it doesn’t involve medication. This is particularly valuable for patients who prefer to avoid or have not responded well to substance-based treatments.
In addition, if patients are already on medication for depression or other conditions, there is no risk of interactions or adverse effects with TMS. This makes TMS more accessible than other options, as it doesn’t require switching medications. While switching antidepressant medications can take months, patients can start TMS immediately.
Another key advantage of TMS is its ability to target a specific area of the brain. As mentioned, the magnetic coils are placed over the dorsolateral prefrontal cortex (DLPFC), a region of the brain associated with mental health disorders. The magnetic pulses only reach a depth of about 1.5cm–3 cm beneath the scalp, which minimizes the impact on surrounding tissue and reduces the risk of serious side effects.
Patients don’t need to recover after TMS sessions. They can attend their sessions and, in most cases, resume their normal activities immediately afterward. It’s perfectly safe for patients to drive to and from their appointments, and there are no restrictions placed upon them.
After multiple clinical trials to test safety and efficacy, the FDA approved rTMS to treat patients with depression in 2008. Results showed that rTMS was effective and well-tolerated, with adverse effects being mild and short-lived.
As discussed above, the FDA approved the novel form of TMS known as Intermittent Theta-Burst Stimulation (iTBS) in 2018. This new protocol was shown to be as effective and well-tolerated as rTMS, with the added advantage of just three-minute sessions compared to the 37 minutes required for rTMS.
The SAINT™ protocol — which combined theta-burst stimulation with fMRI and neuronavigation for precise coil placement — received FDA clearance in 2022 and is now considered the “gold standard” for treating treatment-resistant depression.
Many clinics across the US currently offer TMS. The most common TMS treatment available is still rTMS, but an increasing number of clinics are offering accelerated TMS and iTBS protocols.
Many insurance plans cover rTMS, but depending on your provider and policy, you may need to have tried two to four antidepressant medications and/or therapy with a psychologist or psychiatrist before qualifying for TMS insurance coverage. Since TMS is usually more expensive than most antidepressants, insurance providers typically won't authorize payment for TMS as the first-line therapy.
Currently, the latest and most effective form of TMS — accelerated fMRI-guided theta burst stimulation — is not covered by insurance. However, as this protocol becomes more popular, insurance companies may start to offer coverage.
Though there are considerably more advantages than disadvantages of TMS, there are some potential downsides that patients should be aware of.
Some patients may experience pain and discomfort during or after TMS sessions. This can include mild scalp discomfort, mild headaches, or muscle contractions. While this may lead some patients to stop treatment, clinical trials show that this affects less than 2% of patients. Those who experience headaches after treatment can use over-the-counter painkillers such as ibuprofen or paracetamol. In most cases, patients become accustomed to the procedure, and the discomfort diminishes after a few sessions.
With rTMS, which involves 30-minute sessions five days per week for four to six weeks, the time commitment can be difficult to fit into patients’ daily routines. Some patients may not complete their treatment due to this, which can reduce the effectiveness of TMS.
This is one of the reasons accelerated one-week protocols like what we offer at Cognitive FX are an attractive alternative for patients.
The most serious side effect during TMS treatment is seizures, but the risk is minimal for most patients. Most of the cases of seizures occurred in the early days of TMS. Since then, many safety features have been added to the procedure, significantly reducing the risk for patients.
Current estimates suggest that less than 3 patients experience a seizure per 100,000 sessions. Patients are more likely to experience seizures during the initial sessions, but they can occur later in the course of treatment, particularly if there are clinical or medication changes.
Seizures are more likely in patients with a history of seizures or suffering from neuropsychiatric diseases, including epilepsy, multiple sclerosis, traumatic brain injury, and Alzheimer’s disease. In addition, patients who routinely experience poor sleep, drink excessively, take prescription drugs that lower the seizure threshold, or are suffering from high levels of stress also have a higher risk of seizures. The good news is that seizures tend to be isolated events and don’t cause serious long-term consequences. There is no evidence that patients may experience multiple seizures during or after TMS sessions.
With fMRI-guided iTBS, the risk of seizure is further reduced because it applies pulses at 80% of the brain’s motor threshold, compared to the high-frequency pulses of 110%–120% used with rTMS, making it even safer for patients.
Most patients can undergo TMS sessions, but there are exceptions. Patients with metallic implants or objects in the brain near the treatment area should not receive TMS. This includes cochlear implants, internal pulse generators, medication pumps, aneurysm clips or coils, stents, and bullet fragments. (Patients with braces and dental fillings can safely undergo TMS.)
TMS poses a risk for these patients because the magnetic stimulation may cause their devices to malfunction. Magnetic pulses can also cause brain implants to overheat, potentially leading to irreversible brain damage. In patients without metal implants, the heating produced during a TMS session is negligible and poses no risk to the patient.
At Cognitive FX, we currently do not treat patients under 18 or over 65 years old, those experiencing suicidal ideation, or those with a history of seizures.
While TMS has demonstrated relatively high success rates, not all patients experience significant improvements or achieve remission. Outcomes also depend on the type of support patients receive.
At Cognitive FX, we incorporate cognitive behavioral therapy (CBT) with TMS for a more holistic treatment approach. We believe this will yield better long-term outcomes for our patients. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. We anticipate CBT will provide similar, if not greater, improvements for patients receiving accelerated fMRI TMS.
Conventional TMS treatments range from $6,000 to $15,000 without insurance. However, costs can vary significantly depending on geographic location, provider expertise, the type of TMS used, and insurance coverage.
Clinics with advanced technology or additional services, such as medical evaluations or talk therapy, may charge higher fees. For example, clinics offering SAINT™ treatment are charging $30,000+.
Note: At Cognitive FX, we offer an alternative to SAINT for a fraction of the price. More on this below.
The use of fMRI and neuronavigation for precise and consistent coil placement throughout treatment is a relatively new practice and is not widely available due to the expense of this technology. At most TMS clinics, the coil’s placement is determined by manually measuring distances between the patient’s nose, ears, and the top of the head. However, due to variations in head size, shape, and individual brain organization, this method can be imprecise and result in less consistent outcomes.
With the use of fMRI and neuronavigation, healthcare practitioners can accurately position the magnetic coil over the dorsolateral prefrontal cortex (DLPFC) for each patient and each session. This factor plays a major role in why the SAINT protocol, which uses this approach, is producing the best TMS treatment outcomes to date.
With its non-invasiveness and targeted stimulation of a specific brain region implicated in depression, TMS is a promising solution for patients who have tried other treatments and failed to experience any benefits. If you’ve tried multiple antidepressant medications without success, it’s worth considering TMS as a treatment for your depression.
In particular, the speed with which accelerated fMRI-guided theta burst stimulation can achieve high response and remission rates, combined with mild side effects, makes it one of the safest and most effective treatments available today.
Our clinic in Provo, Utah, provides an alternative to SAINT™ TMS that offers the same precision of personalized treatment targeting, combined with FDA-approved theta burst stimulation at a significantly lower cost. This approach delivers the same core elements that make SAINT so revolutionary.
The only difference between our treatment and SAINT™ (a trademark licensed to Stanford Medical) is our targeting method. Our target locations are determined by fMRI and our prescribing neuroscientist and physician, rather than their proprietary software.
| Accelerated fMRI - TMS | Magnus SAINT™ TMS | |
|---|---|---|
| FDA-Approved iTBS | ✔ | ✔ |
| FDA-Approved Neuronavigators | ✔ | ✔ |
| FDA-Approved Figure 8 Coils | ✔ | ✔ |
| Number of Treatment Days | 5 | 5 |
| Treatments per Day | 10 | 10 |
| Total Treatments | 50 | 50 |
| Number of TMS Pulses | Approx. 90,000 | 90,000 |
| Resting motor threshold pulse intensity | 90–120% | 90–120% |
| FDA-Approved Personalized DLPFC Targeting | ✘ | ✔ |
| Personalized DLPFC Targeting Assists Doctor in Target Location | ✔ | ✘ |
| Personalized E Field Coil orientation | ✔ | ✘ |
| Cost | $9,000 to $12,000 | $30,000+ |
This accelerated TMS protocol is:
To improve patient outcomes, we also include cognitive behavioral therapy (CBT) as a part of our treatment. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our brain stimulation treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. Furthermore, as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX.

Dr. Mark D. Allen holds a Ph.D. in Cognitive Science from Johns Hopkins University and received post-doctoral training in Cognitive Neuroscience and Functional Neuroimaging at the University of Washington. As a co-founder of Cognitive Fx, he played a pivotal role in establishing the unique and exceptional treatment approach. Dr. Allen is renowned for his pioneering work in adapting fMRI for clinical use. His contributions encompass neuroimaging biomarkers development for post-concussion diagnosis and innovative research into the pathophysiology of chronic post-concussion symptoms. He's conducted over 10,000 individualized fMRI patient assessments and crafted a high-intensity interval training program for neuronal and cerebrovascular recovery. Dr. Allen has also co-engineered a machine learning-based neuroanatomical discovery tool and advanced fMRI analysis techniques, ensuring more reliable analysis for concussion patients.
If you’re considering accelerated Transcranial Magnetic Stimulation (TMS) therapy, it’s important to understand how it works, what to expect, and whether it’s the right fit for you.

If you’re considering transcranial magnetic stimulation (TMS) therapy, one of the first and most important questions you’re likely to ask is: “Is TMS safe?”

Transcranial magnetic stimulation (TMS) and neurofeedback are gaining popularity as non-invasive, medication-free options for treating depression—especially for people who haven’t found relief from ...

For many people living with major depression, antidepressant medications either don’t work or only get them part of the way to recovery. Symptoms may ease for a while, but then return, or never fully...

If you or a loved one have had failed attempts with traditional antidepressant drugs, you may be considering alternative treatment options such as electroconvulsive therapy (ECT) and transcranial...
Transcranial magnetic stimulation (TMS) has been FDA-approved for treating major depressive disorder (MDD) since 2008 and is a well-established treatment option, especially for patients who haven’t...