While many people are learning about Transcranial Magnetic Stimulation (TMS) for the first time, this brain stimulation method has been helping patients for nearly 40 years. Originally developed as an experimental tool for brain research, TMS is now considered an established FDA-approved treatment for major depressive disorder and other mental health conditions—particularly for patients who haven’t found relief with antidepressant medication or psychotherapy.
This article provides a broad overview of TMS for patients considering the treatment. We cover:
A Brief History of TMS
The roots of TMS go back nearly two centuries. In 1831, English physicist Michael Faraday discovered that a magnetic field can induce an electrical current—a principle known as electromagnetic induction. Over 150 years later, in 1985, British engineer Anthony Barker applied this principle to the brain, creating the first TMS device. His coil-based system could stimulate the motor cortex every five seconds and was used mainly as a research tool.
By the mid-1990s, researchers were testing TMS for neurological and psychiatric conditions, especially major depression. Multiple clinical trials and meta-analyses confirmed its safety and effectiveness, laying the groundwork for broader adoption.
A pivotal moment came in 2008, when the U.S. Food and Drug Administration (FDA) approved the use of TMS for treatment-resistant depression. Since then, TMS has gained worldwide recognition and additional approvals for other conditions.
How TMS Treatment Works
The original TMS protocol, known as repetitive TMS (rTMS), targets the dorsolateral prefrontal cortex (DLPFC)—an area of the brain linked to mood regulation. During treatment, a magnetic coil is placed over the scalp to deliver pulses that modulate brain activity.
The typical rTMS protocol involves:
-
Schedule: 5 sessions per week for 4–6 weeks (20–30 sessions total)
-
Session length: About 40 minutes
-
Pulse pattern: 5–10 seconds of stimulation followed by 30–60 seconds of rest
-
Timeline for improvement: Some patients improve in 2–4 weeks; others notice benefits only after completing treatment
Over the past two decades, rTMS has become widely accepted, and most U.S. health insurance plans now cover it.
Effectiveness & Safety of TMS Therapy
Effectiveness
TMS is proven as an effective treatment option for reducing symptoms of depression:
Maintenance sessions can help preserve results, sometimes for years.
Safety
TMS is non-invasive, well-tolerated, and has a strong safety record, including:
-
Mild side effects: Scalp discomfort, tingling, muscle twitches, mild headache, lightheadedness
-
Rare side effects: GI discomfort, insomnia, tinnitus, fainting
-
Seizure risk: Extremely rare—less than 0.01% per session (under 1 in 10,000)
FDA-Approved Uses
TMS is FDA-approved for:
- Major depressive disorder (treatment-resistant depression)
- Obsessive-compulsive disorder (OCD)
- Migraines
- Smoking cessation
Off-label uses include bipolar disorder, Parkinson’s disease, epilepsy, and post-traumatic stress disorder (PTSD).
Key Advancements to Address the Limitations of rTMS
Since 2008, several new TMS methods have been developed to increase safety and efficacy while addressing key limitations of conventional TMS.
1. Accelerated Protocols (Intermittent Theta-Burst Stimulation)
The need to attend sessions 5 days per week makes conventional rTMS treatment challenging to complete amidst work, childcare, and other life commitments. In 2018, the FDA approved intermittent theta-burst stimulation (iTBS), which condenses sessions from 37 minutes to about 3 minutes.
Some clinics offer iTBS on the same treatment schedule as rTMS. Sessions take a fraction of the time, but patients still need to visit the clinic 5 days per week for 4 to 6 weeks. Other clinics offer accelerated protocols that deliver multiple sessions per day, completing a full course of treatment in as little as 5 days.
2. Targeted Stimulation (fMRI and Neuronavigation)
One key limitation of rTMS lies in how technicians determine where to place the magnetic coil. The most common approach—the “5 cm method”—involves clinicians manually measuring the patient’s head to estimate the correct stimulation site. While simple to perform, it does not account for individual differences in head size, shape, or brain structure. Even a deviation of just a few millimeters from the dorsolateral prefrontal cortex (DLPFC) can significantly reduce treatment effectiveness.
To address this, Stanford researchers developed Stanford Intelligent Accelerated Neuromodulation Therapy (SAINT™), an FDA-approved protocol from 2022 now regarded as the gold standard for treatment-resistant depression.
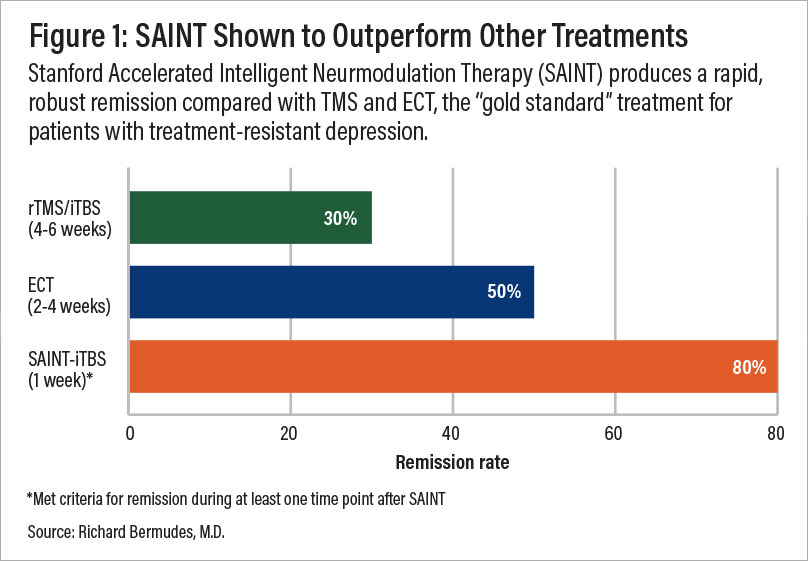
A comparison of remission rates for rTMS/iTBS, electroconvulsive therapy (ECT), and SAINT-iTBS.
SAINT uses functional MRI (fMRI) to pinpoint each patient’s exact DLPFC location based on brain connectivity patterns. During treatment, neuronavigation software ensures the coil is placed over that precise spot for every session, eliminating much of the targeting inconsistency seen with traditional methods.
This precision—combined with an accelerated delivery schedule—has produced remarkable outcomes. The SAINT protocol delivers 10 sessions per day for 5 consecutive days, totaling 50 sessions. Many patients experience rapid and substantial symptom relief.
The Unique TMS Protocol Offered at Cognitive FX
Our Utah-based clinic, Cognitive FX, offers an accelerated fMRI-guided TMS protocol with the same core elements that make SAINT™ TMS so revolutionary— fMRI and neuronavigated targeting with accelerated iTBS delivery—at a significantly lower cost.
Key differences from Magnus SAINT™ TMS:
-
We use advanced fMRI analysis by our neuroscientist and physician, rather than proprietary software, to determine the stimulation site.
-
We process scans in-house, leveraging 25 years of clinical fMRI experience from treating brain injury patients.
-
We pass those efficiencies on to patients: $9,000–$12,000 vs. $30,000+.
To improve outcomes for our patients, we also include cognitive behavioral therapy (CBT) as part of our treatment. When combined with the traditional TMS (rTMS) method, CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Eligibility & Next Steps
Our outpatient, non-invasive treatment is ideal for most patients with major or treatment-resistant depression. For safety, we do not treat:
- Patients under 18 or over 65
- Patients with a seizure history
- Actively suicidal patients needing crisis care
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX. Or, take our quiz to see if you’re a good fit for treatment.
Cited Research