Side Effects of TMS: Do the Benefits Outweigh the Risks?
If you’re considering transcranial magnetic stimulation (TMS) therapy, you may be concerned about its side effects and whether the benefits outweigh any risks.

If you’ve tried multiple antidepressant medications without enough relief, you may be exploring newer depression treatment options for treatment-resistant depression (TRD) or major depressive disorder (MDD). Two FDA-approved options often considered at this stage are Spravato® (esketamine nasal spray) and transcranial magnetic stimulation (TMS).
While both can help reduce depressive symptoms, they work in very different ways, involve different risks, and fit into a treatment plan differently. Understanding these differences can help you make an informed decision with your healthcare provider.
In this guide, we compare Spravato and TMS based on:
TMS therapy is a non-invasive treatment that uses focused magnetic pulses to stimulate specific areas of the brain involved in mood regulation, most commonly the prefrontal cortex. These brain regions are often underactive in people with mental health conditions like depression.
Unlike medications that act chemically throughout the body, TMS directly influences brain activity by stimulating nerve cells involved in emotional regulation.
During a TMS session, a magnetic coil is placed on the scalp. The device delivers brief pulses that create small electrical currents in targeted brain tissue. Over a treatment course, these repeated stimulations help normalize activity in disrupted neural networks.
Standard repetitive TMS (rTMS) typically involves daily sessions over 4–6 weeks. For many, this can be a challenging practical component of completing treatment amidst work and life commitments.
Newer accelerated approaches, including intermittent theta-burst stimulation (iTBS), deliver treatment in much shorter sessions and can produce fast-acting symptom relief. For example, the accelerated fMRI-guided TMS treatment we offer at Cognitive FX is completed in just 5 days.
Most patients feel light tapping or tingling on the scalp. Some experience scalp discomfort or mild headaches early on, which usually fade as treatment continues. Patients remain awake—there is no sedation, anesthesia, or recovery time.
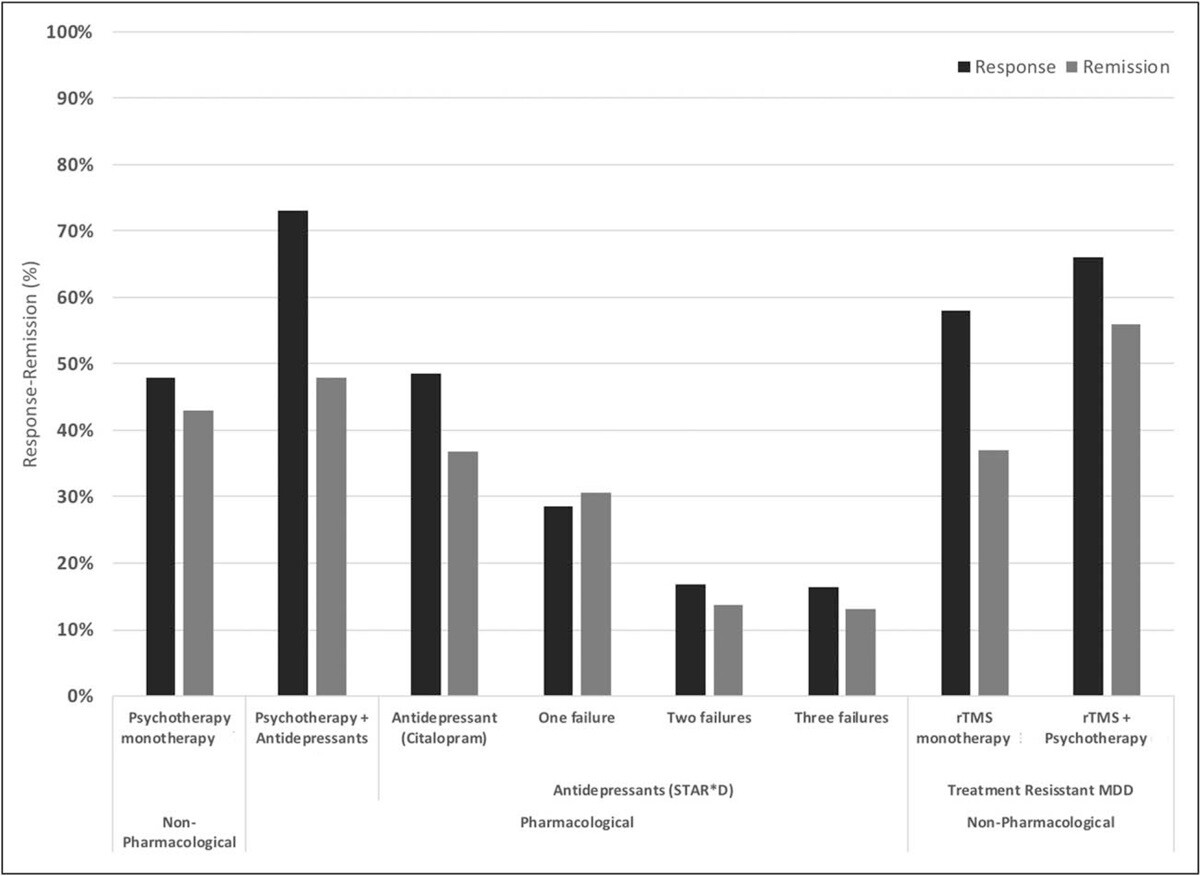
Response and remission rates of various monotherapeutic and combinatory antidepressant treatments based on the largest studies and datasets available. [Source]
Across studies:
~50% of patients respond (i.e. they see some meaningful reduction in depression symptoms) to standard TMS (known as repetitive TMS or rTMS).
~30% achieve remission.
Outcomes improve when combined with psychotherapy with remission and response rates of ~55% and ~66% respectively.
Accelerated and iTBS protocols produce similar or better results, often with rapid relief, including reductions in suicidal ideation for some patients.
When fMRI and neuronavigation are used for precision targeting (an approach known as SAINT™, developed at Stanford University), several clinical trials have shown that effectiveness rates improve further, with about 85% of patients responding to the treatment and around 78% achieving remission within five days of treatment. About four weeks later, remission rates dropped somewhat but remained around 60%.
The SAINT™ protocol received FDA-clearance in September 2022 and is now considered the “gold standard” for treating treatment-resistant depression.
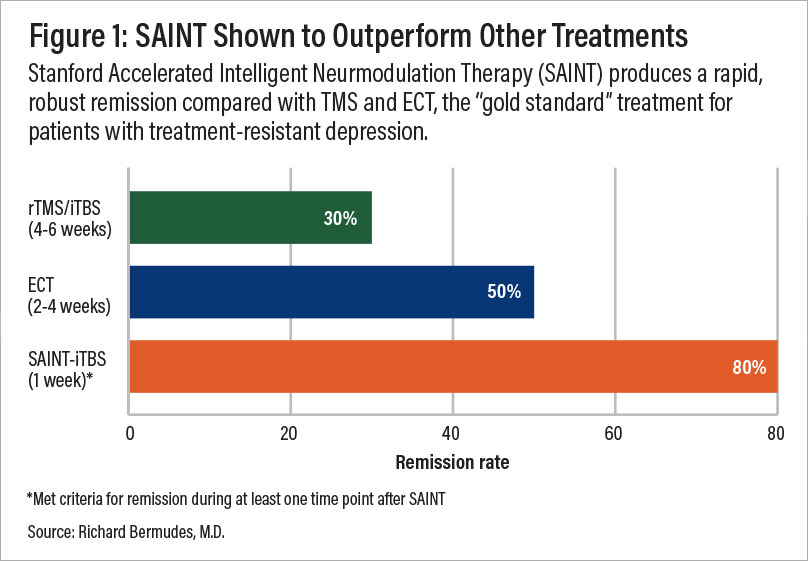
A comparison of remission rates for rTMS/iTBS, ECT, and SAINT-iTBS.
TMS is associated with minimal side effects, most commonly:
The most serious potential side effect—a seizure—is extremely rare (less than 0.01% per session).
The only patients for whom TMS may not be safe are patients with metallic implants (or other objects) in the brain close to where the coil needs to be placed. This includes, for example, cochlear implants, internal pulse generators, medication pumps, aneurysm clips or coils, stents, or even bullet fragments. (Patients with braces and dental fillings can undergo TMS safely.)
A full TMS treatment typically costs $6,000–$15,000. Standard rTMS is often covered by insurance after failure of multiple traditional antidepressants and psychotherapy. Accelerated, personalized protocols are usually self-pay and can run up to $30,000+.
Spravato treatment uses esketamine, a derivative of ketamine, delivered as a nasal spray under strict medical supervision. It is FDA-approved for treatment-resistant depression and for major depressive disorder with suicidal thoughts.
Unlike oral antidepressants that target serotonin or norepinephrine, esketamine acts on NMDA receptors in the brain, increasing activity of the neurotransmitter glutamate. This can rapidly enhance neural connectivity and synaptic signaling involved in mood.
Typically, a Spravato treatment involves two phases:
Week 1 to 4 (induction phase): During this phase, patients take esketamine twice weekly. The first dose is usually 56mg, but it may increase to 84mg in subsequent sessions. At the end of this period, patients discuss with their doctor whether to continue with Spravato or start taking antidepressant medication.
Week 5 and onward (maintenance phase): If side effects are tolerated and symptoms are improving, patients move to a maintenance phase with weekly sessions for a while and then once every two weeks. This should still be combined with antidepressant medication or psychotherapy to reduce the risk of relapse.
Spravato is administered in certified healthcare settings only. After dosing, patients are monitored for at least two hours for changes in blood pressure, alertness, and adverse reactions.
Some patients experience heightened senses (such as seeing vivid colors and hearing loud sounds), dizziness, a sense of detachment (dissociation) from reality, or feeling like they’re drunk. These side effects usually subside within a few hours, and patients slowly return to normal.
Patients cannot drive afterward and should plan for a full day of reduced activity.
Several clinical trials have studied the efficacy of Spravato to treat depression, but the results have been somewhat contradictory: some studies failed to identify any differences in effectiveness rates between esketamine and antidepressant medication, while others found some benefits, but only modest and when used in combination with other antidepressant drugs.
In the long term, esketamine seems to reduce by half the risk of relapse by 32 weeks, but about one fourth of patients experienced symptoms again despite continuing treatment. Suicidal thinking decreased considerably but there was no evidence this occurred exclusively due to esketamine.
While Spravato can provide fast-acting symptom relief—sometimes within hours—benefits often depend on continued dosing. Studies show:
Spravato has a broad list of potential side effects, including:
Rare but serious risks include respiratory depression, cardiovascular events, worsening suicidal ideation, and misuse. Because of these risks, Spravato carries an FDA Boxed Warning and is distributed only through a drug safety program called risk evaluation and mitigation strategies (REMS).
Spravato is not appropriate for all patients.
Absolute contraindications (should not receive Spravato):
Relative contraindications (require careful monitoring and clinical judgment):
Spravato often costs $18,000–$45,000 per year. Many insurance plans cover it for treatment-resistant depression, though prior authorization and proof of failed antidepressant trials are usually required.
|
Feature |
Spravato |
TMS |
|
Mechanism |
Chemical (glutamate) |
Electrical (magnetic pulses) |
|
Speed |
Very fast |
Fast to gradual |
|
Durability |
Requires ongoing dosing |
Can persist after treatment |
|
Side Effects |
Significant, systemic |
Mostly local and mild |
|
Supervision |
Intensive |
Minimal |
|
Invasiveness |
Drug-based |
Non-invasive |
Spravato may be appropriate for patients with severe symptoms or urgent suicidal thoughts, especially when rapid symptom reduction is critical. However, it carries meaningful safety risks and usually requires ongoing treatment.
TMS offers a highly targeted approach with minimal side effects, no systemic drug exposure, and the potential for longer-lasting improvement—particularly when paired with psychotherapy.
In some cases, clinicians may consider both approaches together. Because these treatments affect different systems, combining them may enhance outcomes—but only under close guidance from an experienced clinician.

At Cognitive FX, we offer an accelerated fMRI-guided TMS protocol that combines the core strengths of SAINT™ TMS—precision targeting and an accelerated treatment schedule—without the $30,000+ price tag.
Key differences between our treatment and Magnus SAINT™:
We use advanced fMRI analysis by our neuroscientist and physician, rather than proprietary software, to determine the stimulation site.
We process scans in-house, leveraging 25 years of clinical fMRI experience from treating brain injury patients.
We pass those efficiencies on to patients: $9,000–$12,000 vs. $30,000+.
| Accelerated fMRI - TMS | Magnus SAINT™ TMS | |
|---|---|---|
| FDA-Approved iTBS | ✔ | ✔ |
| FDA-Approved Neuronavigators | ✔ | ✔ |
| FDA-Approved Figure 8 Coils | ✔ | ✔ |
| Number of Treatment Days | 5 | 5 |
| Treatments per Day | 10 | 10 |
| Total Treatments | 50 | 50 |
| Number of TMS Pulses | Approx. 90,000 | 90,000 |
| Resting motor threshold pulse intensity | 90–120% | 90–120% |
| FDA-Approved Personalized DLPFC Targeting | ✘ | ✔ |
| Personalized DLPFC Targeting Assists Doctor in Target Location | ✔ | ✘ |
| Personalized E Field Coil orientation | ✔ | ✘ |
| Cost | $9,000 to $12,000 | $30,000+ |
Our TMS protocol is:
Safe: Widely tolerated and associated with mild, short-lasting side effects. This is in stark contrast with eketamine, which is often associated with dissociation, intoxication, sedation, high blood pressure, dizziness, headache, blurred vision, anxiety, nausea, and vomiting.
Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape and individual brain structure (a key innovation). Neuronavigation ensures the magnetic coil is placed over that exact spot for every treatment session.
Fast: Treatment courses are reduced to a single week, making it easier to complete alongside life and work commitments.
Effective: Precision coil placement combined with theta burst stimulation produces the best TMS treatment results to date.
To improve outcomes for our patients, we include cognitive behavioral therapy (CBT) as part of our treatment. When combined with conventional TMS, CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
Our treatment is ideal for most patients with treatment-resistant depression. However, we do not treat patients under the age of 18 or over 65. And as a safety measure, we do not treat patients who have a history of seizures or who are currently actively suicidal and in need of crisis care.
Click here to learn more about receiving accelerated fMRI TMS therapy at Cognitive FX. Or, take our quiz to see if you’re a good fit for treatment.
If you’ve been researching alternative depression treatments, you may or may not be familiar with transcranial magnetic stimulation and its different variations. If you’d like to learn more, the following articles are a good place to start:

Dr. Mark D. Allen holds a Ph.D. in Cognitive Science from Johns Hopkins University and received post-doctoral training in Cognitive Neuroscience and Functional Neuroimaging at the University of Washington. As a co-founder of Cognitive Fx, he played a pivotal role in establishing the unique and exceptional treatment approach. Dr. Allen is renowned for his pioneering work in adapting fMRI for clinical use. His contributions encompass neuroimaging biomarkers development for post-concussion diagnosis and innovative research into the pathophysiology of chronic post-concussion symptoms. He's conducted over 10,000 individualized fMRI patient assessments and crafted a high-intensity interval training program for neuronal and cerebrovascular recovery. Dr. Allen has also co-engineered a machine learning-based neuroanatomical discovery tool and advanced fMRI analysis techniques, ensuring more reliable analysis for concussion patients.

If you’re considering transcranial magnetic stimulation (TMS) therapy, you may be concerned about its side effects and whether the benefits outweigh any risks.

While antidepressant medications remain the most common first-line treatment for depression, research shows they only work for about one-third of patients and often come with unpleasant side effects...

With traditional antidepressant medications only working effectively for about one-third of patients, researchers and clinicians are continuously developing new treatment options for...

If you or a loved one have had failed attempts with traditional antidepressant drugs, you may be considering alternative treatment options such as electroconvulsive therapy (ECT) and transcranial...

Traditional antidepressant medications involve a trial period of weeks or months before it can be determined whether or not they are working. If a medication doesn’t work — and it’s been shown that ...

Transcranial magnetic stimulation (TMS) and neurofeedback are gaining popularity as non-invasive, medication-free options for treating depression—especially for people who haven’t found relief from ...