If you’re exploring non-invasive brain stimulation depression treatments, you may be considering Transcranial Magnetic Stimulation (TMS) and Magnetic e-Resonance Therapy (MeRT—a specialized form of TMS).
This article explains how they compare, shared limitations of these approaches, and how newer accelerated fMRI-guided TMS protocols address those gaps to provide more effective treatment outcomes.
We cover:
A Brief Overview of TMS vs. MeRT Therapy
Transcranial magnetic stimulation (TMS) is a non-invasive treatment that uses magnetic pulses to stimulate nerve cells in brain regions associated with depression and other mental health conditions. By restoring more typical patterns of neural activity, TMS can lead to lasting relief from depressive symptoms — often with success rates that exceed those of antidepressant medication and with fewer side effects.
The most common form of TMS therapy is repetitive transcranial magnetic stimulation (rTMS), which was first approved by the FDA in 2008. The success of rTMS has driven the development of newer approaches, including MeRT, which uses quantitative electroencephalography (qEEG) to personalize treatment parameters with the goal of improving outcomes.
Key Differences Between TMS and MeRT Treatment
1. Personalization and Treatment Targets
Standard TMS
Conventional TMS follows a fixed treatment protocol targeting a specific area of the brain called the left dorsolateral prefrontal cortex. While stimulation intensity is often individualized, the target location and treatment frequency are largely standardized across patients.
MeRT
The primary distinction between TMS and MeRT is that MeRT uses quantitative EEG (qEEG), sometimes alongside an electrocardiogram (ECG), to measure a patient’s specific brainwave patterns before and during treatment. These data are used to guide decisions about stimulation frequency and coil placement, with the goal of synchronizing stimulation with the individual’s unique brain rhythms.
In addition to targeting regions associated with depression, MeRT is designed to address broader patterns of dysregulation by stimulating different brain regions over the course of treatment to support overall brain network connectivity.
2. Scientific Evidence
The scientific evidence supporting TMS and MeRT varies significantly in depth and level of formal recognition.
TMS
TMS is supported by decades of rigorous research and is widely recognized as a standard clinical treatment for depression. Numerous controlled trials and meta-analyses have demonstrated its safety and efficacy, leading to broad clinical adoption.
MeRT
MeRT is a more recent application of TMS and does not yet have the same level of extensive clinical validation. While it uses FDA-cleared TMS equipment, the specific MeRT protocol is considered off-label or experimental for many conditions. Large-scale, peer-reviewed trials are still needed to determine whether MeRT offers meaningful advantages over standard TMS.
3. Treatment Timelines
|
Therapy
|
Typical Duration
|
TMS Session Length
|
|
rTMS
|
4–6 weeks
|
~30–40 minutes
|
|
MeRT
|
6–8 weeks
|
45+ minutes
|
|
Accelerated TMS
(discussed below)
|
~5 days
|
Multiple short sessions per day
|
4. Conditions Treated
TMS
TMS is used to treat a limited number of conditions, most notably Major Depressive Disorder (MDD), Obsessive-Compulsive Disorder (OCD), anxious depression, and smoking cessation.
MeRT
MeRT is often marketed for a broader range of neurological and psychiatric concerns, though the strength of evidence varies significantly by condition. These commonly include autism, ADHD, PTSD, Traumatic Brain Injury (TBI), concussions, dementia, insomnia, brain fog, and stroke.
5. Regulatory Status
TMS
TMS is FDA-approved for the treatment of Major Depressive Disorder, Obsessive Compulsive Disorder (OCD), and smoking cessation. It is also commonly used in patients with anxious depression.
MeRT
While MeRT uses FDA-cleared equipment, the specific MeRT protocol is often considered off-label and experimental. Some clinics advertise MeRT as FDA-cleared, but this designation applies only to the equipment, not the protocol itself. At present, the MeRT protocol is not FDA-approved for the treatment of depression.
6. Cost and Insurance Coverage
|
Factor
|
TMS
|
MeRT
|
|
Per-session cost
|
~$100–$300
|
~$200–$500
|
|
Insurance coverage
|
Often covered (with treatment-resistant criteria)
|
Usually self-pay
|
|
Additional testing
|
Minimal
|
Repeated qEEG/analysis fees
|
Related reading:
Similarities Between TMS and MeRT
Despite their differences, both therapies share a common foundation. They:
- Use magnetic stimulation delivered through a coil placed on the scalp
- Are non-invasive and require no anesthesia
- Allow patients to resume normal activities immediately after treatment
- Have generally mild side effects, such as scalp discomfort or headache
Serious risks, such as seizure, are rare and screened for during evaluation.
Comparing Effectiveness Rates
-
Standard rTMS produces meaningful symptom improvement in approximately 50–60% of patients, with remission rates around 30%.
-
Response rates are typically lower in individuals with highly treatment-resistant depression.
-
Early studies suggest MeRT may achieve comparable outcomes to rTMS, but evidence remains limited.
fMRI-guided accelerated TMS protocols have shown significantly higher remission rates in research and clinical settings, though availability and cost can vary.
Shared Limitations of Standard TMS and MeRT
Whether used in isolation or with a qEEG, TMS is a safe and worthwhile depression treatment to consider, especially with its effectiveness rates and mild side effects compared to most other treatments.
However, standard TMS and MeRT have two important limitations:
1. Lengthy Treatment Courses
Traditional treatment schedules require daily visits for 4 to 8 weeks, which can be difficult to complete while managing work, family, or severe symptoms.
2. Indirect Targeting Methods
Many clinics still rely on manual, scalp-based measurements (such as the 5-cm method) to position the magnetic coil. Because head anatomy varies, this approach often leads to imprecise targeting. Studies show that missing the treatment target area by a few millimeters can significantly reduce treatment effectiveness.
Adding EEG data with the MeRT protocol does not necessarily solve this spatial-targeting challenge, since coil placement is still translated from scalp measurements rather than direct brain imaging.
For more detail on the limitations of qEEG, read this article.
How Accelerated fMRI-Guided TMS Solves These Issues to Produce Better Treatment Outcomes
Stanford Intelligent Accelerated Neuromodulation Therapy (SAINT™), which was FDA-approved to treat major depressive disorder (MDD) in 2022, addresses both of these issues.
First, it uses functional MRI to map brain connectivity and identify an individualized treatment target, along with neuronavigation to position the magnetic coil precisely over that target during every treatment session.
Second, the SAINT protocol uses an accelerated form of TMS known as intermittent theta burst stimulation (iTBS). This approach condenses treatment into a single week, making it easier for patients to fit into their lives while offering faster symptom relief. Treatment consists of 10 brief sessions per day over 5 days, with approximately 50 minutes between sessions.
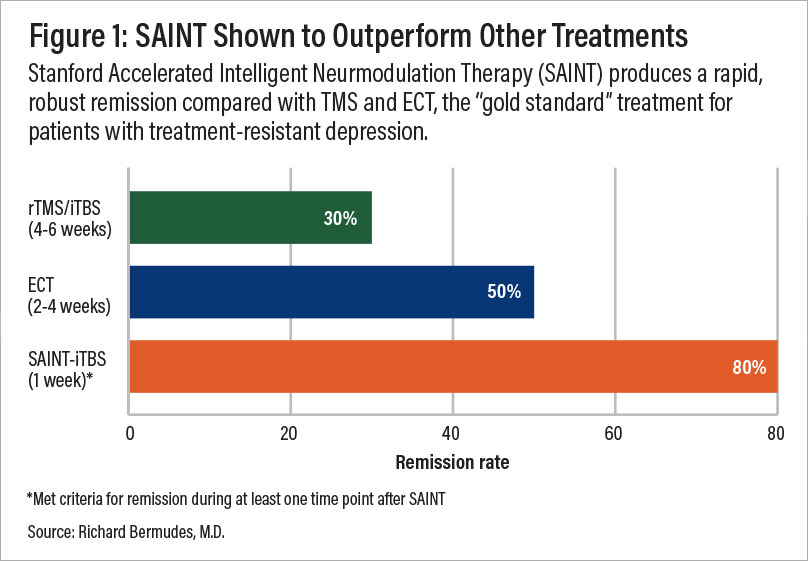
A comparison of remission rates for rTMS/iTBS, ECT, and SAINT-iTBS.
Following this protocol, patients experienced significant reductions in depressive symptoms and suicidal ideation within five days, with no serious adverse effects reported. In a double-blind, randomized controlled trial, about 86% of patients responded to the treatment (meaning they met prespecified criteria for reduced depressive symptoms) and approximately 79% met the remission criterion.
All participants had treatment-resistant depression and had failed at least two prior depression treatments. One month after the clinical trial, 60% of patients remained in remission. (Find the full text of the sham-controlled study here.)
The speed with which SAINT treatment can achieve high response and remission rates, combined with minimal side effects, makes it one of the best fast-acting depression treatments available today.
With that said, some practical barriers make SAINT treatment less accessible:
-
Lack of insurance coverage: With the exception of Medicare and Medicaid coverage in certain hospital-based settings, most insurance plans do not yet cover SAINT. However, coverage policies vary, so it’s important to check with your insurance provider to confirm your options.
-
High treatment costs: Currently, SAINT TMS treatments range from $30,000 to $36,000, making the cost a significant barrier for many patients. There are, however, close alternatives available at a significantly lower price (discussed below).
-
Limited availability: Few clinics in the U.S. currently offer SAINT, with most providers still using rTMS. However, more clinics are beginning to adopt accelerated TMS and intermittent theta burst stimulation (iTBS) protocols, signaling a gradual shift toward newer treatment methods.
A More Affordable SAINT™ Alternative: Accelerated fMRI-Guided TMS at Cognitive FX
Our clinic in Provo, Utah offers an accelerated fMRI-guided iTBS protocol that combines the core strengths of SAINT™—personalized treatment targeting and an accelerated treatment schedule—without the $30,000+ price tag.
Key differences from Magnus SAINT™ TMS:
-
We use advanced fMRI analysis performed by our neuroscientist and physician to determine stimulation targets, rather than relying on proprietary software.
-
fMRI scans are processed in-house, leveraging 25 years of clinical fMRI experience treating patients with brain injury.
-
These efficiencies allow us to offer treatment at a significantly lower cost, approximately $9,000–$12,000 compared to $30,000+.
This protocol of TMS is:
-
Safe: Widely tolerated and associated with mild, short-lasting side effects.
-
Precise: fMRI ensures that the treatment target area is precisely located for each patient, accounting for variations in head size and shape. Neuronavigation ensures the magnetic coil is positioned over that same target for every treatment session.
-
Fast: Treatment is delivered over a single week, making it easier to complete alongside work and daily life (compared to the 4–6 weeks required for standard TMS protocols).
-
Effective: Precision coil placement combined with theta burst stimulation produces the best TMS treatment results to date.
To improve outcomes for our patients, we also include cognitive behavioral therapy (CBT) as a part of our treatment plan. When combined with the traditional method of TMS (rTMS), CBT improved response and remission rates by ~8% and ~19%, respectively. Additionally, CBT is likely to produce sustained improvement over time once treatment has concluded.
See If You’re a Good Fit
Our treatment is ideal for most treatment-resistant patients, as well as those looking for alternative drug-free depression treatments. However, we do not treat patients under the age of 18 or over 65. Additionally, as a safety measure, we do not treat patients who have a history of seizures or are actively suicidal and in need of crisis care.
Take our quiz to see if you’re a good fit for receiving accelerated fMRI TMS therapy at Cognitive FX or call our patient coordinator at 385-334-6093 to learn more.
Cited Research